* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Transplantation Immunology
Monoclonal antibody wikipedia , lookup
Molecular mimicry wikipedia , lookup
Major histocompatibility complex wikipedia , lookup
Adaptive immune system wikipedia , lookup
Psychoneuroimmunology wikipedia , lookup
Innate immune system wikipedia , lookup
Polyclonal B cell response wikipedia , lookup
Cancer immunotherapy wikipedia , lookup
Adoptive cell transfer wikipedia , lookup
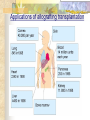
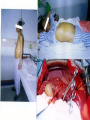
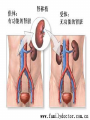
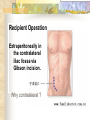
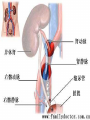
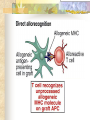
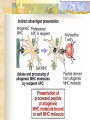
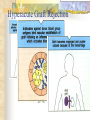
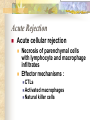
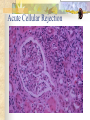
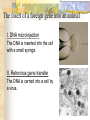
Transplantation Xiang Li, Urology Department West China Hospital, Sichuan University Acknowlegement To Dr. Lu Yiping and Dr. Wang jia To other Colleagues working on renal and liver transplantation Transplantation is a Dream? Dream of Paranoia Dream of excellent surgeon who wants to excel himself. Dream of excellent scientist who believe nothing is impossible. Can you imagine? Can you imagine? Can you imagine? Contents Basic concepts of transplantation Clinical Organ transplantation Renal Transplantation, RT Transplantation Immunology MHC and Tissue Matching Graft Rejection Immunosuppression Definition of Transplantation Implantation of „non-self” tissue into the body the process of taking cells, tissues, or organs called a graft (transplant), from one part or individual and placing them into another (usually different individual). donor : the individual who provides the graft. recipient or host: the individual who receives the graft. Blood Transfusion First attempts were unsuccesful (MISMATCH) Discovery of blood groups (Red cell antigens) A-B Landsteiner 1900 Rh Levine, Stetson 1939 Succesful transfusion = Transplantation Others: Bone, Tissue-engineering, etc Transplantation Organ Transplantation Classification of Renal Transplantation Auto-RT Cadaveric Allograft RT Living related Living Donor Living unrelated Xenograft RT (In experimental) Transplantation History experimental kidney transplantation -1912 Alexis Carel-Nobel prize 1935 human kidney transplant in Russia - rejection P.B. Medawar (1945) skin grafts Self skin accepted Relative not accepted ! What is the difference ? Immunologic mechanism A. Mitchison (1950) Lymphocytes are responsible for rejection Transplantation History Peter Gorer (~1935) Identification of 4 group of genes for RBC Gorer and Gorge Snell (~1950) Group II antigens are responsible for rejection Major HistoCompatibility genes (HLA) Nobel prize 1980 George Snell 1954 Succesful kidney transplant between identical twins in Boston – Peter Bent Brigham Hospital Joseph Murray 1991 Nobel prize HISTORY OF THE RT 1933 1954 1958 1959 First clinical RT (Voronov); First long-term successful RT(Twin); Discovery of HLA(Human Lym Antigen); Radiation be used for immunosuppression; 1961 Azathioprine (Aza); 1962 Prednisolone; Tissue Matching; 1966 Cross-Matching; Late 1960’ Preservation the Kidney>24hr ; 1972 First successful RT(LRD) in china; 1978 Clinical use of Cyclosporine(CsA). Key factors for succesful transplantation Knowledge of MHC haplotypes Effective immunosuppression Ability to identify and treat infections Available donors Applications of allografting transplantation The importance of transplantation: Clinical Organ Transplantation Liver Transplantation Renal Transplantation LIVER TRANSPLANTATION Indication: End stage liver diseases (ESLD) Hepatic Disease to ESLD Congenital malfomation; Congenital liver metabolic disorders; Acute liver failure; Chronic liver failure: (1) Cirrhosis: Hepatitis B, Alcoholic; (2) Parasites: Hydatid disease of liver, ect. liver malignance RENAL TRANSPLANTATION END STAGE RENAL DISEASES (ESRD) Definition: (1) Various causes; (2) Irreversible injury; (3) Functional failure. Morbidity Europe: 50/million; China: 90-100/million TREATMENT OF ESRD DIALYSIS Chronic Ambulatory Peritoneal Dialysis (CAPD); Hemodialysis (HD). KIDNEY TRANSPLANTATION Renal Transplantation Renal transplantation is associated with as survival benefit for patients with ESRD when compared to dialysis; Even marginal donor kidneys confer a significant survival advantage over maintenance dialysis. The preferred therapy for most of the Pts with ESRD; More cost- effective; Better survival; Better life quality. CONTRAINDICATION Active invasive infection; Active malignance; High probability of operative mortality; Unsuitable anatomic situation for technical success; Severe psychological or financial problem. Pre-OP Selection ABO Blood Group: Compatible; Cytotoxicity Test: Donor Lymphocyte Recipient Serum Cross matching Donor Lymphocyte Donor Serum Recipient Serum Recipient’s Lymphocyte Mixed Lymphocyte Culture Tissue typing (HLA) OPERATION DONOR (1) Living donor Nephrectomy via flank approach; Nephrectomy via Laparoscope. (2) Cadaveric Donor Total midline incision; in situ flashing: Euro-collins/UW solution; Bilateral radical nephrectomy. Low temperature preservation. Potential Advantages of living versus cadaveric kidney donor Better short-term result(about 95% versus 90 % 1-yr function); Better long-term results(half-life of 12-20 yr versus 8-9 yr); More consistent early function and easy of management; Potential Advantages of living versus cadaveric kidney donor Avoidance of brain death stress; Minimal incidence of delayed graft function; Avoidance of long wait for cadaveric transplant; Potential Advantages of living versus cadaveric kidney donor Capacity of time transplantation for medical and personal convenience; Immunosuppressive regime may be less aggressive; Help relieve stress on national cadaver donor supply; Emotional gain to donor. Potential disadvantages of live donation Psychological stress to donor and family; Inconvenience and risk of evaluation process(i.e., intravenous contrast); Operative mortality(about 1 in 2000 Pts.); Major post operative complications (about 2% of Pts.); Potential disadvantages of live donation Minor postoperative complications(up to 50% of Pts.); Long-term morbidity(possible mild hypertention and proteinuria); Risk for traumatic injury to remaining kidney; Risk for unrecognized covert chronic renal disease. Recipient Operation Extraperitoneally in the contralateral iliac fossa via Gibson incision. Why contralateral ? RECIPIENT OPERATION Blood Vessel Anastomosis: Donor renal V Recipient’sexternal iliac V Donor renal A Recipient’s internal iliac A Ureter Anastomosis: Donor ureter Recipient’s bladder Anti-reflux anastomosis Clinical phases of rejection 1. Hyperacute rejection (minutes to hours) 2. 3. Accelerated rejection Acute rejection (around 10 days to 30 days) 4. Preexisting antibodies to donor HLA antigens Complement activation, macrophages Cellular mechanism (CD4, CD8, NK, Macrophages) Chronic rejection (months to years !!) Mixed humoral and cellular mechanism CHRONIC REJECTION IS STILL HARD TO MANAGE ! IMMUNOSUPPRESSION Immunosuppresents play a very important role in organ transplantation; Immuosuppresents extremely increase the effect and the survival rate of organ transplantation; IMMUNOSUPPRESSION Immunosuppresents are a double edged sword; the most important thing is to increase their positive effects, and in the same time decrease their side effects (i.e., organ toxicity, infection, tumors, ect.). Diagnosis of rejection Symptom/Sign fever; urinary output ; graft tenderness; graft size ; hypertension; myalgia/arthragia. Laboratory Test Serum creatine, SCr; Urinary creatine, Ucr; Color doppler scan; radiorenogram; Ateriogram; Biopsy: (1) Fine needle aspiration biopsy (FNAB); (2) Core needle biopsy(CNB). Treatment of kidney rejection Hyperacute (Sometimes during the operation !) No therapy, usually results in graft failure – kidney should be removed Acute (Most frequently in the first 4 weeks) BIOPSY ! Increase immunosuppression Increase steroid dose Increase cyclosporin (monitor serum level !) ATG, ALG, OKT3 Chronic ACE inhibitors, prostacyclin analog drugs Steroid, Imuran, Cellcept Transplantation Immunology Histocompatibility Antigens Major histocompatibility antigens MHC class I molecules : almost all nucleated cells MHC class II molecules : APCs, endothelium of renal arteries and glomeruli Minor histocompatibility antigens : HY molecule Major histocompatibility antigens Human leukocytic Antigen HLA I. (α1, α2, β1, β2) Gene-Code alleles: DP, DQ, DR loci HLA III. Gene-Code alleles: A, B, C loci HLA II. (α1, α2, α3, β2-microglobulin) Gene-Code alleles: C4A, TNF, HSP70 MHC complex: Gene Major histocompatibility antigens MHC loci are highly polymorphic Many alternative alleles at a locus The loci are closely linked to each other A set of alleles is called a HAPLOTYPE One inherites a haplotype from mother and another from father The alleles are codominantly expressed Inheritance of MHC alleles A/C A/D B/C Mother Father A/B C/D B/D A/R1 R2/C R2/R1 Possible children of parents with HLA haplotype A/B and C/D R1=C-D recombination R2=A-B recombination Induction of Immune Responses Against Transplants alloantigens and xenoantigens : antigens that serve as the targets of rejection the antibodies and T cells that react against these antigens are said to be alloreactive and xenoreactive, respectively. allorecognition direct indirect Rejection Senzitization stage Not needed in hyperacute reactions ! Effector stage Alloantibodies: bind to endothelium, activate the complement system, and injure graft blood vessels Rejection Effector stage (Mostly cellular mechanism) Alloreactive T cells : recruit and activate macrophages ---> DTH response delayed type hypersensitivity Alloreactive CTLs: CTL mediated cytotoxicity lyse graft endothelial and parenchymal cells directly ADCC CD4, CD8 lymphocytes, NK cells, macrophages Rejection From: Kuby: IMMUNOLOGY (fourth edition, 2000) Tissue typing Microcytotoxicity assay Known antibody to WBCs of donor / recipient Complement mediated lysis if Ab present on cell surface Mixed lymphocyte culture (MLC) Irradiated donor lymphocytes (stimulants) Incubated with recipient lymphocytes 3H Thymidin incorporatin measured Flow cytometry cross typing DNA analysis Genomic typing (very precise, many subtipes) Hyperacute Rejection Occurs within minutes of transplantation Pre-existing IgM (natural) antibodies against ABO blood group antigens less well-characterized antigens in xenograft alloantigen, such as foreign MHC molecules, or alloantigen expressed on vasular endothelial cells Hyperacute Graft Rejection Acute Rejection Occurs within days or weeks after transplantation Acute vascular rejection Necrosis of cells of the graft blood vessels (vasculitis) Mediated by IgG antibodies endothelial cell alloantigens complement activation against and Acute Rejection Acute cellular rejection Necrosis of parenchymal cells with lymphocyte and macrophage infiltrates Effector mechanisms : CTLs Activated macrophages Natural killer cells Acute Cellular Rejection Chronic Rejection Occurs over months or years Fibrosis with loss of normal organ structures Wound healing following the cellular necrosis A form of chronic DTH or a response to chronic ischemia caused by injury to blood vessels Prevention and Treatment of Allograft Rejection1 Immunosuppression drugs that inhibit or kill T lymphocytes toxins that kill proliferating T cells antibodies that deplete or inhibit T cells anti-inflammatory agents Prevention and Treatment of Allograft Rejection2 Reduce the immunogenicity of allografts ABO blood group typing HLA typing and matching induce donor-specific tolerance blood transfusion Graft-versus-host Disease (GVHD) Occurs in bone marrow recipients Initiated by T cell recognition of host alloantigens The effector cells are less well defined : NK cells, CD8+ CTLs, cytokines Acute GVHD Epithelial cell necrosis : Skin Liver The gastrointestinal tract Characterized by skin rash, jaundice and diarrhea Acute GVH Immunosuppressive therapy 1. Allogenic transplantation always require immunosuppressive therapy Most of the drugs available are nonspecific Common side effects of therapy: Infection Cancer Bone-marrow depression Immunosuppression 2. Conventional drugs (1st phase) Steroids /Prednisone/ (0.1-10 mg / kg) Azathioprine /Imuran/ (0.5-3 mg/ kg) Cyclophosphamide (0.5-20 mg/ kg) Methotrexate (0.1-0.3 mg/ kg) Immunosuppression 3. New drugs (1): CYCLOSPORIN A (=REVOLUTION !) 2-8 mg/ kg FK506 tacrolimus /PROGRAF/ Sirolimus - rapamycin Gusperimus - dezoxyspergualin Effects of cyclosporins Receptor: cytoplasmic immunophillin calcineurin blockage NF-Atc Rapamycin also binds to immunophillin, but the complex does not block calcineurin, it blocks proliferation in G1 phase. Highly lymphocyte specific. IL-2 action is impaired. T lymphocyte (Th) is blocked. Immunosuppression 4. Purin antagonists IMURAN CELLCEPT (micophenolate mofetil) Mizoribin – bredinin Pirimidin antagonists Sodium-brequinar (highly lymphocyte specific) Monoclonal antibodies OKT3 (Anti CD3 mAb) Anti-TAC (anti-IL2 receptor) CD3 T cells Activated T lymphocytes Anti-CD4 Anti-LFA1 + anti-ICAM-1 – experimental Anti-cytokine (IL-2, TNFα, IFNγ) Problems of Transplantation There are not enough organs At least 150,000 patients in industrially developed countries badly need donor organs and tissues Every 14 minutes another name is added to the national transplant waiting list. About 16 people die because of the lack of available organs for transplant each day. Rejection: When the immune system of the host detects foreign graft tissue, it launches an attack, resulting in tissue rejection Gene technology may as a solution Gene technology offers the possibility to breed the desired organs in animals. Lack of organs is no longer a problem Gene technology makes it possible to humanize the bred organs - the immune system identifies the organ as its own tissue. Immune system rejection is prevented From which animals are we able to transplant organs 1. The Chimpanzee: Its DNA sequence differs from ours by only 2% 2. The Baboon: Its organs are too small for a large adult human 3. The Pig: Surprisingly similar to our anatomy and physiology Organ breeding: •A transgenic animal carries a foreign gene inserted into its genome. •The transgenic animal shows the specific characteristics which are coded on the inserted gene A gene which is responsible for the construction of a human organ makes the organism produce the organ additionally. The insert of a foreign gene into an animal I. DNA microinjection The DNA is inserted into the cell with a small syringe II. Retrovirus gene transfer The DNA is carried into a cell by a virus. Suppression of immune system rejection The genes which are responsible for the own tissue not being rejected can be injected into an animal embryo the organs of which are then similar to the ones of the human. It is possible to humanize the bred organs by making certain genetic modifications. Then the organs are accepted by the immune system. Conclusion Transplantation provides us the means of restoring the function of a nonfunctional organ. In the case of BMT it enables us to administer such high doses of chemotherapy that would destroy the BM as well as the residual tumor. A lot immunologic knowledge had to be collected to understand what is happening. Conclusion HLA typing and matching is essential for allografting transplantation. Effective immunosuppressive therapy (Cyclosporin) revolutionised organ transplantation. The future is to transplant cells, that would restore the function of the affected organ. Gene therapy is growing, and will cause another revolution like cyclosporin did in the 1980s. To seek what everybody has sought To think what nobody has thought Try your best, everything will be possible