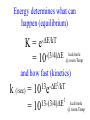* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download H Why - Yale University
X-ray photoelectron spectroscopy wikipedia , lookup
Chemical equilibrium wikipedia , lookup
Franck–Condon principle wikipedia , lookup
Marcus theory wikipedia , lookup
Thermodynamics wikipedia , lookup
Rutherford backscattering spectrometry wikipedia , lookup
Physical organic chemistry wikipedia , lookup
Internal energy wikipedia , lookup
George S. Hammond wikipedia , lookup
Computational chemistry wikipedia , lookup
Bioorthogonal chemistry wikipedia , lookup
Molecular dynamics wikipedia , lookup
Hypervalent molecule wikipedia , lookup
Self-assembly of nanoparticles wikipedia , lookup
Chemical thermodynamics wikipedia , lookup
Energy applications of nanotechnology wikipedia , lookup
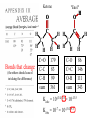
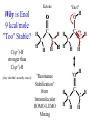
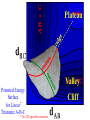
Chemistry 125: Lecture 37 December 8, 2010 Statistics / Equilibrium / Rate: Boltzmann Factor, Entropy, Mass Action, Transition State Theory The values of bond dissociation energies and average bond energies, when corrected for certain “effects” (i.e. predictable errors) can lead to understanding equilibrium and rate processes through statistical mechanics. The Boltzmann factor favors minimal energy in order to provide the largest number of different arrangements of “bits’ of energy. The slippery concept of disorder is illustrated using Couette flow. Entropy favors “disordered arrangements” because there are more of them than there are of recognizable ordered arrangements. The Law of Mass Action explains the exponentiation of concentration in equilibrium equations. Equilibrium ideas allow understanding reaction rates by using transition state theory instead of considering all trajectories on the potential energy surface. This semester’s study of structure and energy leads to next semester’s study of reaction mechanisms and synthesis. For copyright notice see final page of this file Exam Help Sessions Friday, Dec. 10, 10:30-11:30 am (here) Sunday, Dec. 12, 3-5 pm Monday, Dec. 13, 7-9 pm Wednesday, Dec. 15, 8-10pm Thursday, Dec. 16, 7-9 pm Bond Strengths in CH4 Heat of Atomization of CH4 = 397.5 kcal mol-1 (from heat of combustion, Chupka, etc.) Average Bond Energy = 397.5 / 4 = 99.4 kcal mol-1 Individual Bond Dissociation (from spectroscopy etc.) Energies CH3 -H 104.99 ± 0.03 CH2-H 110.4 ± 0.2 CH-H101.3 ± 0.3 C-H Sum 80.9 ± 0.2 No individual bond actually equals the “average” C-H bond. (because of changes in hybridization, etc.) from Barney Ellison (good experiments!) 397.5 ± 0.6 & his friends ve Bond Energies “2nd C-O bond” 90-93 kcal/mole! (Carbonyl group very “stable”) “3rd bond” 54 kcal/mole “2nd C-C bond” 63 kcal/mole (p bond even weaker, since sp2-sp2 s bond is stronger) How good are these average bond energies for calculating "Heats of Atomization” for other molecules? From Streitwieser, Heathcock, & Kosower HAtomization by Additivity of Average Bond Energies? Seems Pretty Impressive! Ave. Bond Energy (kcal/mole) 83 99 146 86 111 C-C C-H C=C C-O O-H S Bond Energies H Error -4.3 -0.8 -5.9 -0.4 atomization kcal/mole Error % Ethene 0 4 1 0 0 542 537.7 c-Hexane 6 12 0 0 0 1686 1680.1 c-Hexanol 6 11 0 1 1 1784 1778.6 -5.4 -0.3 a-Glucose 5 7 0 7 5 2265 2248.9 -16.1 -0.7 12.5 -29.5 -68.4 -240.7 How accurate must you be to be useful? ~10× better than Kcalc = 10-(3/4)(Hcalc) Kcalc = 10-(3/4)(Htrue + Herror) Kcalc = Ktrue 10-(3/4)(Herror) kcal error not % error composition determines K error factor To keep error less than 10 need <1.3 kcal/mole error! Ketone "Enol" O O ve Bond Energies H H C C C H H H H H H H C C C H H H C=O 179 C-O 86 Bonds that change C-C 83 C=C 146 (the others should cancel in taking the difference) C-H 99 O-H 111 sum 361 sum 343 Can one sum bond energies to get -(3/4) 18 -13.5 Kcalc = 10 = 10 accurate"Heats of Atomization"? Kobs = 10-7 = 10-(3/4) 9.3 Ketone Why is Enol 9 kcal/mole "Too" Stable? C(sp2)-H stronger than C(sp3)-H (they shouldn’t actually cancel) "Enol" •• O O H H C C C H H H H H H H C C C H H H C=O 179 C-O 86 C-C 83 C=C 146 +O H "Resonance C-H 99 O-H 111 Stabilization” sum 361 sum C 343 from H H Intramolecular Kcalc = 10-(3/4) 18 =C10-13.5 C HOMO-LUMO -7 H-(3/4)H9.3 H Kobs = 10 = 10 Mixing “Constitutional Energy” from bond additivity needs correction * for effects such as: • Resonance •• HO C H H H vs. C C sp sp H H H (HOMO/LUMO) • Hybridization • Strain CH2 2 3 * Polite name for error in simplistic scheme Energy determines what can happen (equilibrium) -E/kT e K= -(3/4)E = 10 kcal/mole @ room Temp and how fast (kinetics) ‡ 13 -E /kT 10 e k (/sec) = 13-(3/4)E = 10 ‡ kcal/mole @ room Temp What's so great about low energy? Statistics Gibbs 1902 1902 Exponents & Three Flavors of Statistics 1) The Boltzmann Factor 2) The Entropy Factor 3) The Law of Mass Action OnConsidered the Relationship the between the Second Law implications of random of energyand ofdistribution Thermodynamics among realCalculation atoms. Probability regarding the oflaws “I am conscious beingof Thermal Equilibrium only an individual struggling Ludwig Boltzmann 1844 - 1906 weakly against the stream of time. But (1877) it still remains in my power to contribute in such a way that when the theory of gases is again revived, not too much will have to be rediscovered.” S = k ln W Random Distribution of 3 “Bits” of Energy among 4 “Containers” How many “complexions” have N bits in the first container? N 3 2 # 1 3 Random Distribution of 3 “Bits” of Energy among 4 “Containers” How many “complexions” have N bits in the first container? N 3 2 1 # 1 3 6 Random Distribution of 3 “Bits” of Energy among 4 “Containers” How many “complexions” have N bits in the first container? N 3 2 1 0 # 1 3 6 10 30 in 20 30 bits of energy in 20 molecules 3 bits of energy in 4 “molecules” N 3 2 1 0 # 1 3 6 10 Eave = 1/2 kT Boltzmann Constant 1.987 cal/moleK (N) (E) (Note: temperature is average energy) -E/kT e E If all “complexions” for a given Etotal are equally likely, Boltzmann limit shifting energy to any oneshowed degree ofExponential freedom of any one molecule isfor disfavored. reducing the energy available lots of By infinitesimal energy bits elsewhere, this reduces the number of relevant complexions. Exponents & Three Flavors of Statistics 1) The Boltzmann Factor 2) The Entropy Factor 3) The Law of Mass Action Disorder and Entropy "It is the change from an ordered arrangement to a disordered arrangement which is the source of the irreversibility.” The Feynman Lectures on Physics, Vol. I, 46-7 Disorder and Entropy Which is more ordered? Disorder, Reversibility, & Couette Flow Click for webpage and "Magic" movie Couette Flow Syrup If disorder is in the eye of the beholder, how can it measure a fundamental property? Top View Ink line The rotated state only seemed to be disordered. “A disordered arrangement” seems to be an oxymoron. The situation favored at equilibrium has particles that have diffused every whichaway. “A disordered arrangement” is code for a collection of random distributions whose individual structures are not obvious. It is favored at equilibrium, because it includes so many individual distributions. Entropy is Counting in Disguise. Free Energy & 1.377 entropy units K = e-G/RT e-(H - TS)/RT e-H /RT e TS/RT -H /RT S/R e e e-H /RT e R ln 2/R e-H /RT e ln 2 Y e-H /RT x 2 1.377 e.u. (R ln 2) is a common S. e.g. Conclusions: difference in 1.377 e.u. just means entropy a factor between of two. gauche and K depends on anti T butane because of X X S. H, not Y X G (and S) sometimes obscure what is fundamentally simple.Y Anti Gauche Gauche Exponents & Three Flavors of Statistics “there’s a divinity that shapes our ends” -H/RT R 1)Same The Boltzmann Factor e thing: Hamlet V:2 k is per individual molecule R is per mole (= k NA) from counting random arrangements of a fixed number of energy bits k =W 2) The Entropy Factor eTS/kT from counting W, the number of molecular structures being grouped 3) The Law of Mass Action Cyclohexane Conformers 10.8 7.0 kcal/mole 5.5 few quantum states 0 few "structures" Chair (stiff) many "structures" many quantum states Twist-Boat (flexible) few quantum states Both classical and quantum views suggest a statistical "entropy" factor (of ~7) favoring twist-boat. This reduces the room-temperature Boltzmann "enthalpy" bias of 10-(3/4) 5.5 (= 14,000) in favor of chair to about 2,000. few "structures" Chair (stiff) Experimental Entropy Although we discuss entropy theoretically (in statistical terms), physical chemists can measure it experimentally. The entropy of a perfectly ordered crystalline material at zero Kelvin is zero ( ln 1 ). As the material is warmed it gains entropy in increments of (Heat Absorbed)/Temperature. S = H/T “Floppy” molecules with closely spaced energy levels absorb more energy, and at lower temperatures, and thus gain more S on warming. Cf. Ethane rotation - Lecture 33 K = e-G/RT Exponents & Three Flavors of Statistics 1) The Boltzmann Factor e -H/RT from counting random arrangements of a fixed number of energy bits 2) The Entropy Factor eTS/kT = W weighted from counting W, the number of quantum states being grouped 3) The Law of Mass Action from counting molecules per volume Law of Mass Action Late 1700s : Attempts to assemble. hierarchy of “Affinities” Early 1800s : Amounts [concentration] can shift reaction direction away. from “affinity” prediction. … Mid 1800s : Equilibrium “K” as balance of forward and reverse rates... Law of Mass Action [concentration] 2A [A2] 2 [A] A2 = K [A2] = K [A] 2 Where does the exponent come from? Randomly Distributed “Particles” # Particles # Dimers 50 1 100 9 150 19 200 35 250 59 # of Dimers Randomly Distributed “Particles” Increasing concentration increases both the number number of units and the fraction fraction of units that have near neighbors. [D] = K # of Particles # Particles # Dimers [P] 2 50 1 100 9 150 19 200 35 250 59 Equilibrium, Statistics & Exponents Particle Distribution : Law of Mass Action [A2] = K 2 [A] Energy Distribution : H , Boltzmann Factor -H/RT Ke Counting Quantum States : S S/R Ke Free energy determines what can happen (equilibrium) -G/RT e K= -(3/4)G = 10 Energy & Entropy kcal/mole @ room Temp But how quickly will it happen? (kinetics) Visualizing Reaction Classical Trajectories & The Potential Energy Surface Time-Lapse “Classical” (Molecular Mechanics) faster rotating Too rapidly rotating & Complicated slowly vibrating (for our purposes) Trajectory for non-reactive collision of 13 atoms 6 molecules 40 Dimensions slower (3n + time) by E. Heller Potential Energy Rolling Ball Maps A-B Vibration A-B Distance Potential Energy “Surface” for Stretching Diatomic Molecule A-B Plateau Pass + (Transition State or Transition Structure) Potential Energy Surface for Linear * Triatomic A-B-C * So 2-D specifies structure Valley Cliff Vibration of A-B with distant C spectator Potential Energy Surface for Linear Triatomic A-B-C Vibration of B-C with distant A spectator Slice and fold back Unreactive Trajectory: (A bounces off vibrating B-C) Potential Energy Surface for Linear Triatomic A-B-C C flies away from vibrating A-B Reactive Trajectory Potential Energy Surface for Linear Triatomic A-B-C “classical” trajectory (not quantum) A approaches non-vibrating B-C H3 Surface Henry Eyring (1935) Transition State (“Lake Eyring”) Crazy angle of axes means that classical trajectories can be modeled by rolling marble. H + H-Br “I wanted to catch a little one” John McBride (1973) Studying Lots of Random Trajectories Provides Too Much Detail Summarize Statistically with Collective Enthalpy (H) & Entropy (S) “steepest descent” path (not a trajectory) Slice along this path, then flatten and tip up to create… Transition “State” G Starting Materials Products “Reaction Coordinate” Diagram (for one-step transfer of atom B) Not a realistic trajectory, but rather a sequence of three species each with H and S, i.e. Free Energy (G) Free Energy determines what can happen (equilibrium) -G/RT Since the transition state e (universal) K= -(3/4)G = 10 the velocity is not universal, Velocity and of ts theory is not truly in equilibrium kcal/mole with starting materials, and @ room Temp the theory is approximate. how rapidly (kinetics) ‡ 13 -G /RT 10 e k (/sec) = 13-(3/4)G = 10 ‡ Amount of ts kcal/mole @ room Temp (1953) (2007) (1959) Physical Organic Chemistry Paul D. Bartlett 1907-1997 http://osulibrary.oregonstate.edu/specialcollections/coll/pauling/bond/audio/1997v.1-bookdunitz.html 1939 Jack Dunitz: At the time when I was reading that book I was wondering whether chemistry was really as interesting as I had hoped it was going to be. And I think I was almost ready to give it up and do something else. I didn't care very much for this chemistry which was full of facts and recipes and very little thought, very little intellectual structure. And Pauling's book gave me a glimpse of what the future of chemistry was going to be and particularly, perhaps, my future. The Chemical Bond Is there an Atomic Force Law? Feeling & Seeing Molecules and Bonds Understanding Bonding & Reactivity through H = E How chemists learned to treasure Composition, Constitution, Configuration, Conformation and Energy Some Big Questions: The Chemical Bond Is there an Atomic Force Law? How does science know? Feeling & Seeing Molecules and Bonds Compared to what? Understanding Bonding & Reactivity Were chemical through H =bonds E discovered or invented? How chemists learned to treasure Composition, Constitution, WouldConfiguration, we even have chemical bonds Conformation without our particular chemical history? and Energy End of Lecture 37 Dec. 8, 2010 Copyright © J. M. McBride 2009, 2010. Some rights reserved. Except for cited third-party materials, and those used by visiting speakers, all content is licensed under a Creative Commons License (Attribution-NonCommercial-ShareAlike 3.0). Use of this content constitutes your acceptance of the noted license and the terms and conditions of use. Materials from Wikimedia Commons are denoted by the symbol . Third party materials may be subject to additional intellectual property notices, information, or restrictions. The following attribution may be used when reusing material that is not identified as third-party content: J. M. McBride, Chem 125. License: Creative Commons BY-NC-SA 3.0