* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download No Slide Title
Menstrual cycle wikipedia , lookup
Mammary gland wikipedia , lookup
Xenoestrogen wikipedia , lookup
Adrenal gland wikipedia , lookup
Congenital adrenal hyperplasia due to 21-hydroxylase deficiency wikipedia , lookup
Hormonal breast enhancement wikipedia , lookup
Hormone replacement therapy (male-to-female) wikipedia , lookup
Hyperandrogenism wikipedia , lookup
Neuroendocrine tumor wikipedia , lookup
Breast development wikipedia , lookup
Hyperthyroidism wikipedia , lookup
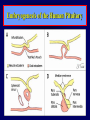
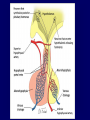
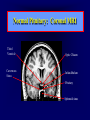
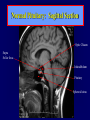
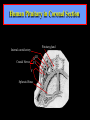
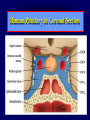
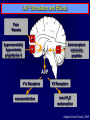
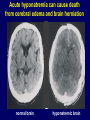
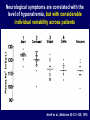
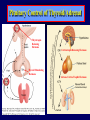
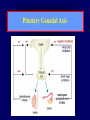
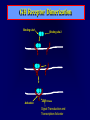
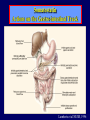
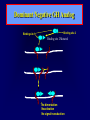
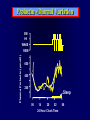
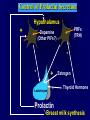
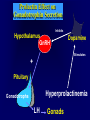
Diseases of the Pituitary Gland Benjamin Glaser, M.D. Endocrinology and Metabolism Service Hadassah-Hebrew University Medical Center Pituitary Disease • Goals of lectures: – At the conclusion of the lectures and seminars (sadnaot) on the pituitary gland, the student should be able to: • Describe the gross anatomy of the pituitary gland, and discuss the relevance of the gland's embryology and anatomy to the physiology and pathology of the pituitary. • Describe the normal control of secretion of vasopressin (ADH). – Describe the normal control of secretion of anterior pituitary hormones. • Discuss the symptoms, clinical signs, laboratory evaluation and treatment of: » » » » Diabetes insipidus Acromegaly Prolactinoma Panhypopituitarism Human Pituitary in Sagittal Section Posterior Clinoids Anterior Clinoids Carotids Third Ventricle Pineal Body Hypothalamus Anterior Commissure Optic Chiasm Pituitary Mamillary Body Median Eminence Normal Pituitary: Gross anatomy Stalk Anterior Posterior The normal gross appearance of the pituitary gland removed from the sella turcica is shown here. The larger portion, the anterior pituitary (adenohypophysis), is toward the top. The image at the left shows the superior aspect of the pituitary with the stalk coming from the hypothalamus entering it. The inferior aspect of the pituitary is shown at the right. The posterior pituitary (neurohypophysis) is the smaller portion at the bottom. Embryogenesis of the Human Pituitary Hypothalamic releasing hormones , ADH Normal Pituitary: Coronal MRI Third Ventricle Cavernous Sinus Optic Chiasm Infundibulum Pituitary Sphenoid sinus Normal Pituitary: Sagittal Section Optic Chiasm Supra Sellar fossa Infundibulum Pituitary Sphenoid sinus Human Pituitary in Coronal Section Pituitary gland Internal carotid artery III IV VI Cranial Nerves V Sphenoid Sinus Human Pituitary in Coronal Section Diseases of the Pituitary • Structural disorders – Trauma - stalk section – Surgery – Tumor • Functional – Hormone Producing • Non-functional – May affect hormone production • Functional disorders – Increased or decreased section of hormones • LH (Leutinizing Hormone) • FSH (Follicle Stimulating Hormone • ACTH (Adrenocorticotrophic Hormone) • TSH (Thyroid Stimulation Hormone) • Growth Hormone • Prolactin Pituitary Gland Hormones Thyroid Gland TSH Vasopressin (Anti-diuretic Hormone) ACTH Adrenal Gland LH FSH Testes/ Ovaries Oxytocin GH IGF-1 Prolactin Vasopressin Biosynthesis Gene expression Preprohormone SP AVP NP GP Prohormone Production Cell body in paraventricula or Supraoptic nucleus AVP Packaging NP GP Glycosylation Proteolysis Amidation AVP Mature Hormone Transport and Maturation Storage and release in posterior pituitary SP, signal peptide; AVP, arginine vasopressin; NP, neurophysin; GP, glycoprotein Receptor-mediated effects of AVP Receptor Subtype Site of Action Activation Effects V1a - vascular smooth muscle cells - platelets - lymphocytes and monocytes - liver vasoconstriction platelet aggregation cytokine release glycogenolysis V1b - anterior pituitary ACTH and endorphin release - renal collecting duct principal cells free water reabsorption V2 Lee et al., Am Heart J 146:9-18, 2003 AVP Collecting Duct Cell AQP3 H2O ATP GTP (Gs) AVP V2 Receptor AQP2 cAMP Exocytic Insertion PKA AQP2 AQP4 Basolateral membrane Recycling vesicle Thyroid hormone Glucocorticoid Dependent Endocytic Retrieval Luminal membrane Collecting duct Vasa recta AVP regulation of water reabsorption from renal tubular cells H2O Renal Concentrating Mechanism Dilute Cortex 10+ Liters/day Na H2O 300 400 Medulla H2O Na H2O 800 ADH Present H2O 1100 Modified from Schrier, Renal and Electrolyte Disorders, Na Concentrated Renal Diluting Mechanism Dilute Cortex 10+ Liters/day Na H2O 400 Medulla H2O H2O Na 500 ADH Absent Na 600 Modified from Schrier, Renal and Electrolyte Disorders, Dilute AVP Stimulation and Effects Pain Nausea + hyperosmolality hypovolemia angiotensin II baroreceptors natriuretic peptides – + AVP V1a Receptors vasoconstriction V2 Receptors renal H2O reabsorption Adapted from Orlandi, 2009 ADH Secretion ADH Plasma Levels ADH levels increase Markedly as soon as The plasma osmolality increases about 285 270 280 300 290 Plasma Osmolality 310 Goldilocks Approach to Endocrinology Goldilocks Approach to Endocrinology Syndrome of Hyperfunction Too Hot Inappropriate ADH Diabetes Hypofunction Too Cold Insipidus Normal JustFunction right Diabetes Insipidus ADH Deficiency Diabetes Insipidus (ADH Deficiency) • Symptoms: – Polyuria (>2,000 cc/d) • If pituitary, usually sudden onset – Polydipsia • If pituitary, usually ice cold water • Signs – – – – Dehydration Hypernatremia Normokalemia Inappropriately dilute urine Diabetes Insipidus • Differential Diagnosis – Osmotic diuresis (e.g. glucose) – Excessive fluid intake • Psychogenic • Central • Secondary – Nephrogenic Diabetes Insipidus – Central Diabetes Insipidus (ADH deficiency) Differential Diagnosis of Nephrogenic Diabetes Insipidus (partial list): • Genetic • Chronic renal disease – – – – Polycyctic disease Pylonephritis Ureteral obstruction Advanced renal failure • Electrolyte disorders – Hypokalemia – Hypercalcemia • Drugs – Alcohol – Lithium – Many others • Miscellaneous – – – – Multiple myeloma Amyloidosis Sjoger's disease Sarcoidosis Differential Diagnosis of Central Diabetes Insipidus • Pituitary tumor – – – – – Functioning (Prolactin, Growth Hormone etc) Non-functioning Craniopharyngioma Dysgerminoma Metastatic tumor (breast, lung) • Trauma – Surgery – Head trauma • Inflammation – Infundibulo-hypophysitis – Granulomatous disease (histiocytosis X, Sarcoid, Tuberculosis) • Genetic Diabetes Insipidus: Clinical Evaluation • Water deprivation test: – Complete fast – Measure hourly urine and serum electrolytes, orthostatic blood pressure and weight – When plasma osmolarity ≥ 295 or urinary osmolarity is stable, then give • DDAVP 5mcg. s.c. – Interpretation: • Lack of ability to concentrate urine - DI • Good response to DDAVP - Central DI • No response to DDAVP – Nephrogenic DI Central Diabetes Insipidus Treatment • Desmopressin (1-desamino-8-D-arginie vasopressin) – Commercial names: DDAVP, Minerin – Long-acting ADH analog • Intra-nasal -- 10-40 mcg ever 8-12 hours • Oral: 0.05 - 0.4 mg every 8-12 hours • IV/SC: 1-2 mcg every 8-12 hours – Monitor: • Electrolytes -- SIADH Syndrome of Inappropriate ADH Syndrome of Inappropriate ADH (SIADH) • Clinical findings: – Hyponatremia – Normokalemia – Euvolemia (mild volume expansion) SIADH - Water and Sodium Balance • Increased ADH activity – Decreased free water clearance – Increased total body water • Hyponatremia • Increased ECF volume • Increased ECF volume – – – – Increased GFR Decrease proximal nephron Na+ reabsorption Increased sodium loss Minimizing increased ECF volume • No edema – Worsening hyponatremia Prevalence of dysnatremias at initial presentation to a health care provider (data from 303,577 samples on 120,137 patients available for analysis) 30 Acute hospital care Ambulatory hospital care Community care 28.2 25 Prevalence (%) 21 20 15 10 7.2 5 0 1.43 0.49 0.17 0.03 Na < 116 Na < 135 0.53 0.72 Na > 145 0.06 0.01 0.01 Na > 165 Hawkins. Clin Chim Acta 337:169-172, 2003 Symptomatic hyponatremia: neurological manifestations • • • • • • • • • headache irritability nausea/vomiting mental slowing confusion/delerium disorientation stupor/coma convulsions respiratory arrest symptomatic but less impaired; usually chronic life-threatening, usually acute Acute hyponatremia can cause death from cerebral edema and brain herniation normal brain hyponatremic brain Neurological symptoms are correlated with the level of hyponatremia, but with considerable individual variability across patients Arieff et al., Medicine 55:121-129, 1976 Plasma Vasopressin (pg/mL) Plasma AVP levels are inappropriately elevated in most patients with SIADH 11 10 9 8 7 6 5 4 3 2 1 0 Normal Range 230 240 250 260 270 280 290 300 Plasma Osmolality (mOsm/kg) 310 Robertson et al. Am J Med 72:339-353, 1982 Causes of SIADH Pulmonary Disorders Acute respiratory failure Infections Positive-pressure ventilation Tumors Extrathoracic Mediastinal Pulmonary SIADH Drugs Carbamazepine Phenothiazines Chlorpropamide Prostaglandin-synthesis Clofibrate inhibitors Cyclophosphamide SSRIs Desmopressin MAO inhibitors Nicotine Tricyclics Oxytocin Vincristine Opiates CNS Disorders Acute psychosis Hemorrhage Inflammatory and demyelinating diseases Mass lesions Stroke Trauma Miscellaneous HIV infection Idiopathic Pain Postoperative state Prolonged exercise Senile atrophy Severe nausea Syndrome of Inappropriate ADH (SIADH) • Diagnosis: – Hyponatremia – Inappropriately elevated urine sodium – No volume depletion or severe volume expansion • Etiology: – – – – – Glucocorticoid deficiency Hypothyroidism Pulmonary lesions CNS lesions Drugs (Chlorpropamide) • Treatment: – Glucocorticoid/Thyroid hormone replacement (if indicated) – Water deprevation – V2 receptor antagonists (in clinical trials) Anterior Pituitary Gland Axes Thyroid Gland TSH ACTH Adrenal Gland LH FSH Testes/ Ovaries GH IGF-1 Prolactin Distribution of Endocrine Cells in Pituitary Thyrotropin Releasing Hormone Posterior Pituitary Dopamine PRL 15% GH 50% Stimulator of secretion Inhibitor of secretion LH FSH 10% Gonadotropin Releasing Hormone LH FSH 10% PRL 15% ACTH 20% TSH 5% GH 50% Corticotropin Releasing Hormone Growth Hormone Releasing Hormone Somatostatin (Somatotropin Release Inhibiting Factor) Pituitary Control of Thyroid/Adrenal Thyrotropin Releasing Hormone Thyroid Stimulating Hormone Corticotropin Releasing Hormone Adreno-Cortico Trophic Hormone Pituitary Gonadal Axis Loss of Pituitary Function Loss of Pituitary Function • Functional abnormalities – – – – – – Gonadotrophins -- Gonadal Insufficiency ACTH -- Adrenal Insufficiency Thyroid -- Hypothyroidism GH – Growth hormone deficiency Prolactin -- No syndrome Anti-diuretic hormone -- Diabetes Insipidus • Structural abnormalities – Visual field disturbance – Cranial nerve dysfunction – CNS leak Loss of Pituitary Function: Etiology • Congenital • Pituitary tumors – Functional – Non-functional • Non-pituitary tumors – Craniopharyngioma – Metastases • Trauma – Surgical – Head trauma • Inflammation – Autoimmune hypophysitis – Granulomatous disease • Histiocytosis X • Sarcoid • Tuberculosis – Rathke’s pouch rupture Anterior Pituitary Function Tests (Stimulation tests) • • • • Thyrotrophin Releasing Hormone (TRH) Gonadotrophin Releasing Hormone (GnRH) Metyropone stimulation test GH-specific tests – Clonidine, Arginine, Exercise, L-dopa • Insulin-induced hypoglycemia (ITT, IST) • Combined pituitary function test – ITT + TRH + GnRH Hormone Replacement Therapy in Panhypopituitary Patient • Adrenal Cortex: – – – – Dexamethasone Prednisone Hydrocortisone Cortosone Acetate 0.25 - 0.75 mg/d 5-7.5 mg/d 15-30 mg/d 25-37.5 mg/d • Thyroid: – Levothyroxin 100-200 mcg/d • Maintain T4 level in upper normal range • Gonadal Steroids: – Estrogen/Progesterone or Testosterone • Desmopressin • Growth Hormone Hypopituitarism -- Treatment • Treatment: – Hormonal Replacement – Surgical • Most tumors require surgery – Radiation • • • • Small effect High probability of pituitary dysfunction Low probability of secondary tumor May have long-term subtle neurologic effects – Medical • Steroids for hypophysitis • Specific treatment for granulomatous disease • Rarely responsive to bromocriptine or octreotide Growth Hormone Growth Hormone (GH) • Gene: – Chromosome 17 q 22-24 – 191 amino-acids • Protein – Species specific – Binds to 2 specific plasma proteins • Low affinity, high capacity -- significance not known • High affinity -- identical to extracellular domain of GH receptor – Absent in GH-receptor deficient states (Laron Dwarf) – Decreased in GH excess – Biologic significance » Decreased clearance? » Buffer effect? • Receptor – Highly species specific Diurnal Variation in Pulsatile Growth Hormone Secretion Regulation of GH Secretion Sleep Arginine Dopamine ? Acetylcholine Somatostatin Glucose TRH Alpha adrenergic Opiates GABA SS GRH GH T3 Stimulation Inhibition Growth Hormone Releasing Hormone + IGF-1 Insulin-like Growth Factor 1 Growth-Hormone: Regulation of Secretion • Growth Hormone Releasing Hormone (GRH) – 40 AA peptide, structurally similar to Glucagon and Secretin – Secreted in pulsitile manner – Interacts with somatostatin to create normal pulsitile GH secretion pattern – Continuous stimulation with GRH results in down-regulation of receptors, but pituitary still responsive – GRH-stimulated GH secretion can be inhibited by Somatostatin Growth-Hormone: Regulation of Secretion • Somatostatin – 2 isoforms: 14 and 28 Amino Acids synthesized from same pro-hormone molecule – Pulsitile secretion with biologic half-life 1-3 minutes – Suppresses both GH and TSH – Produced in hypothalamus, brain, pancreatic islets, GI tract – At least 5 different receptor sub-types – In hypothalamus acts as neuropeptide, secreted by hypothalamic neurons and carried to pituitary by portal system Somatostatin - Structure NH2 COOH Prepro-somatostatin 116 COOH Pro-somatostatin COOH Somatostatin - 28 28 Somatostatin -14 COOH 14 Somatostatin -Cellular Action Ca++ close K+ Somatostatin 14 Somatostatin 28 5 Receptor Subtypes 1 3 Phospholipase C 2 4 Tyrosine phosphatase 5 a Gi AC ATP cAMP GH Receptor Dimerization Binding site 1 Activation Binding site 2 JAK Kinase Signal Transduction and Transcription Activator Insulin-Like Growth Factor 1 • 70 Amino Acids, structurally similar to Proinsulin • GH stimulates secretion. • Blood levels decreased by starvation and liver disease • Most GH-sensitive tissues produce I GF-1 which acts in a paracrine fashion. • Circulating IGF-1 primarily of hepatic origin • Circulating IGF-1 is bound to at least 6 different IGF binding proteins. – IGFBP-3 is regulated by GH Growth Hormone Actions • Anti-Insulin – – – – Lipolysis Ketogenesis Hyperglycemia Peripheral insulin resistance • Growth promoting, Insulin-like – Increased: • Protein synthesis • Amino acid transport • Muscle mass • Bone and cartilage growth • Cell proliferation Growth-Hormone Deficiency (1) • Children – Increased insulin sensitivity • Hypoglycemia (mostly infants and small children) – Decreased linear growth (Dwarfism) – Decreased muscle mass, increased fat mass • Adults – Decreased muscle mass, increased fat mass – Decreased sense of well being Growth-Hormone Deficiency (2) • Etiology: – Children: • Genetic: – – – – Isolated GH deficiency Growth hormone mutations GH receptor deficiency (Laron Dwarf) Multiple pituitary hormone deficiency (PIT-1, PROP-1) • Tumor: – Craniopharyngioma – Dysgerminoma – Adult: • Trauma / Surgery / Radiation • Tumor: – Functioning or non-functioning pituitary – Craniopharyngioma – Metastatic (breast) Growth-Hormone Deficiency (3) • Diagnosis – Stimulation tests: • • • • • Clonidine L-dopa Arginine Exercise Insulin-induced hypoglycemia – Under most circumstances, at least 2 abnormal tests required for definitive diagnosis GH Stimulation Tests Sleep Arginine Dopamine Acetylcholine Alpha adrenergic (Clonidine) Stress (Exercise, ITT) SS GRH GH Stimulation Inhibition + IGF-1 Growth-Hormone Deficiency (4) • Treatment – Human growth hormone replacement • Human pituitary origin – Expensive and insufficient supplies – Creutzfeld-Jacob disease • Recombinant DNA technology – Plentiful – Safe – Expensive – Use in GH-deficient adults standard in most countries – Use in elderly is controversial and not accepted by most. Growth-Hormone Excess • Childhood – Gigantism • Adults – Acromegaly • Etiology – 98%: GH-producing pituitary tumor – 2%: Ectopic GHRH secretion • • • • Small cell lung cancer Bronchial or intestinal carcinoid tumors Pancreatic islet cell tumor Pheochromocytoma Gigantism 28 October 2013, Turkey Growth-Hormone Excess • Childhood – Gigantism • Adults – Acromegaly • Etiology – 98%: GH-producing pituitary tumor – 2%: Ectopic GHRH secretion • • • • Small cell lung cancer Bronchial or intestinal carcinoid tumors Pancreatic islet cell tumor Pheochromocytoma Acromegaly (1) • • • • • Prevalence: Incidence: Mean age of onset: Mean age at diagnosis: Prognosis: 40-50 / 106 3-4 / 106 32 years 42 years 2x increased mortality Acromegaly - Signs and Symptoms • GH Excess – Enlargement of hands and feet – Thick skin – Skin tags – Sweating – Sleep Apnea – Carpal Tunnel Syndrome – Glucose intolerance – Osteoarthritis – Colonic Polyps • Tumor-related – Headache – Visual field defect – Loss of pituitary function • Gonadotrophins • TRH - hypothyroid • ACTH - Addison’s Acromegaly (3): Diagnosis • Abnormal net GH secretion over time: – 24 hour GH profile (Night-time GH levels) – Elevated IGF-1 – Elevated IGFBP-3 • Non-suppressible GH secretion – Glucose suppression • OGTT • High-carbohydrate meal Regulation of GH Secretion Dopamine Glucose TRH SS GRH GH Stimulation Inhibition + IGF-1 Acromegaly Treatment Options • Surgery • Radiation • Medical Natural Somatostatin • Clinical utility is limited by: – Plasma half- life 1-3 minutes • Drug delivery: – Intravenous only – Specificity poor • Cessation of treatment associated with rebound phenomenon Synthetic Somatostatin Analogue Octreotide • Clinical utility is enhanced due to: – Plasma half- life 1-2 hours – Drug action Variable 3-12h – Drug deliveryIV or SC • Specificity: – Partial selectivity • (GH>glucagon>gastric acid>insulin) • No rebound phenomenon upon cessation of treatment Somatostatin Analogs Native Somatostatin-14 S-S Ala-Gly-Cys-Lys-Asn-Phe-Phe-Trp-Lys-Thr-Phe-Thr-Ser-Cys S-S Octreotide: dPhe-Cys-Phe-dTrp-Lys-Thr-Cys-Thr(ol) 111In-DTPA S-S Octreoscan: dPhe-Cys-Phe-dTrp-Lys-Thr-Cys-Thr(ol) Lanreotide: S-S dnal-Cys-Tyr-dTrp-Lys-Val-Cys-Thr Somatostatin Actions on the Gastro-intestinal Track Lamberts et al NEJM, 1996 Adverse Side-Effects of Somatostatin Analogs • Common side-effects – Pain at injection site – Abdominal pain and cramps – Diarrhea, steatorrhea – Impaired glucose tolerance – Gallstone formation • Rare side-effects – – – – Rash Alopecia Water intoxication Hypoglycemia Decrease side-effects by: Avoid fat intake for first 2 weeks after initiation of therapy Short acting preparations: Warm injection to room temperature before administration Inject slowly Use highest possible concentration of drug Inject about 1 hour after meals Acromegaly: Novel Treatment Approaches • Growth hormone antagonists – Growth hormone analog with one binding site mutated Dominant Negative GH Analog Binding site 1 Binding site 2 Binding site 2 Mutated No dimerization Noactivation No signal transduction Acromegaly: Novel Treatment Approaches • Growth hormone antagonists – Growth hormone analog with one binding site mutated – Dominant negative activity – Disadvantages: • GH levels not suppressed • Difficult to monitor • Effect on tumor unknown – May actually stimulate tumor growth Prolactin Prolactin • 198 Amino acid peptide structurally related to Growth Hormone (GH) • Lactotrophs which make up 40-50% of the endocrine cells of the anterior pituitary • During fetal development, prolactin cells appear to differentiate from GH cells. • Some cells maintain the ability to produce both GH and Prolactin. • Glycosylated and non-glycosylated forms have different bioactivities. Cellular Action of Prolactin • Membrane bound receptor • Action appears not to be cAMP dependent • Action may be mediated through membrane phospholipase activity resulting in protein kinase C activation and calcium influx Effects of Hyperprolactinemia • Hypothalamus: – Inhibition of GnRH production • Mammary gland: – Direct - stimulation of milk production and secretion – Indirect - decreased estrogen effect • Ovaries: – Direct - decreased responsiveness to gonadotrophins – Indirect - decreased gonadotrophin secretion at level of hypothalamus/pituitary Effects of Hyperprolactinemia • Testes: – Direct - decreased responsiveness to gonadotrophins – Indirect - decreased gonadotrophin secretion at level of hypothalamus/pituitary • Bones: – Indirect - due to gonadal steroid deficiency Prolactin - Diurnal Variation SW I+II WAKE Plasma Prolactin (pmol/l) REM 600 400 200 Sleep 08 14 20 02 24 Hour Clock Time 08 Control of Prolactin Secretion Hypothalamus + PRFs (TRH) Dopamine (Other PIFs?) - + Lactotropes - Estrogen Thyroid Hormone Prolactin Breast milk synthesis Prolactin Effect on Gonadotrophin Secretion Inhibits Hypothalamus GnRH Dopamine Stimulates + Pituitary Hyperprolactinemia Gonadotrophs LH Gonads Differential Diagnosis of Hyperprolactinemia • Prolactin producing pituitary tumor – Microprolactinoma (<1 cm) – Macroprolactinoma (>1 cm) – Mixed tumors (30% of GH producing tumors) • Chronic renal failure – Decreased clearance and suppressibility • Thoracic sensory nerve stimulation – Chest wall burns, incisions, trauma etc. • Mental and physical stress – May be mediated through ß-endorphin suppression of dopamine secretion Differential Diagnosis of Hyperprolactinemia • Medications – Alpha-methyldopa, reserpine – Phenothiazines, butyrophenones, – benzamides (metoclopramide, sulpride) Estrogens – H2-receptor blockers (cimetidine) – Opiates • Hypothyroidism • Decreased dopamine delivery to pituitary – Pituitary, suprasellar and hypothalamic lesions – Radiation damage to the hypothalamus Differential Diagnosis of Hyperprolactinemia • Prolactin levels > 6,500 mIU/l is usually indicative of macroprolactinoma. • Stalk compression, medications, hypothyroidism and stress usually result in prolactin levels < 1,500 and virtually always less than 5,000 mIU/l. • Microprolactinomas, mass lesions compressing the pituitary stalk frequently present with similar prolactin levels. Prolactinoma: Results of Treatment Response Recurrence Surgery Microprolactinoma Macroprolactinoma Radiotherapy Medical Therapy Microprolactinoma Macroprolactinoma 60-80% 10-30% Normalization of PRL after ~10 years >90% 50-80% 50% ~100% TheEndo End The Schematic View of an ADH-Sensitive Collecting Tubule Cell Adenyl cyclase ATP ATP kinase cAMP cAMP ADH receptor H2O A B C H2O Cortisol and Thyroid Hormone requiring ADH binds to the contraluminal surface, activating adenylyl cyclase and generating cAMP. This causes cytoplasmic tubules containing water channels, aquaporins (A), to fuse with the luminal membrane (B), allowing free transport of water into the cell. C = particle aggregates in luminal membrane. Control of ADH Secretion Osmoreceptor (preoptic nucleus anterior hypothalamus) Water Na Glucose Nausea Pain Paraventricular nucl. Supraoptic nucl. Depolarization Posterior Pituitary ADH+ Neurophysin (1.5 hours) Baroreceptrors Aorta Carotids Atria Thoracic veins Acromegaly: Treatment options Transsphenoidal Surg. Radiotherapy Micro Octreotide Bromocriptine Macro GH <5 mcg/l 80% 50-60% 77% (15 years) 65% 20% GH <2 mcg/l 70% 40% no data 40% no data Nl IGF-1 50% no data 50% 10% Late response inconv. & cost Low efficacy 50% Disadvantages Recurrence 5-10%* Complications Hypopit. 15% >50% None None Other DI- 2-3% Neuro deficits Gallstones Nausea, hypotens. * Actual long-term recurrence probably higher