* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Pituitary Function and Pathology
Hypothalamic–pituitary–adrenal axis wikipedia , lookup
Hormonal breast enhancement wikipedia , lookup
Hyperthyroidism wikipedia , lookup
Hypothyroidism wikipedia , lookup
Graves' disease wikipedia , lookup
Hypothalamus wikipedia , lookup
Hyperandrogenism wikipedia , lookup
Growth hormone therapy wikipedia , lookup
Kallmann syndrome wikipedia , lookup
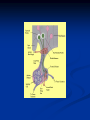
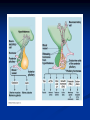
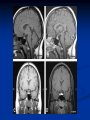
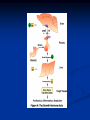
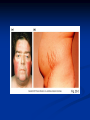
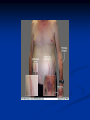
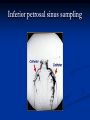
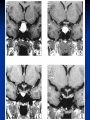
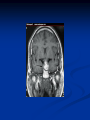
Pituitary Function and Pathology Dr Duncan Fowler The Ipswich Hospital Overview Anatomy Physiology Assessment of pituitary function: static and dynamic tests Clinical scenario’s: Cushing’s Disease Acromegaly Prolactinoma Apoplexy Learning Objectives Describe pituitary anatomy and endocrine physiology Describe methods for assessing pituitary function using static and dynamic testing Describe the new standard for the measurement of growth hormone & its effects on clinical criteria Be are of the importance of screening for macroprolactin Hypothalamo-pituitary anatomy Hypothalamus is the part of the diencephalon associated with visceral, autonomic, endocrine affective and emotional behaviour Ventral portion forms the infundibulum Posterior to this is the median eminence – the final point of convergence of pathways from the CNS on the endocrine system and is vascularised by the primary capillaries of the hypothalamohypophyseal portal vessels Sella turcica Terminology Adenohypophysis = anterior pituitary controlled by releasing and inhibiting factors released from nerves in the median eminence into the hypophyseal portal vessels which carry them to the pituitary Neurohypophysis = posterior pituitary. It is an extension of the CNS. Its function is controlled by direct neural connection to the hypothalamus Presentation of Pituitary Disease Hormonal hypersecretion e.g. Acromegaly Hormonal deficiency e.g amenorrhoea These can occur with or without: Local pressure effects: headaches, visual field loss – bitemporal hemianopia – bump into things Sensitivity of the axes to damage With tumours and radiotherapy GH and gonadal axes are more likely to be affected early with thyoroid and adrenal axes less susceptible (one reason why pituitary tumours present earlier in women) With lymphocytic hypophysitis the opposite is the case Lymphocytic hypophysisitis Mainly occurs in women in late pregnancy or 1st year after delivery May be autoimmune (linked with Hashimoto’s thyroiditis) Posterior pituitary not affected Can cause mass effect (enhances on MRI) Life threatening ACTH deficiency can occur Biopsy for definitive diagnosis if required Variable natural history Identical chain but specific chain – non covalently associated Luteinising hormone (LH) Follicular stimulating hormone (FSH) Thyroid stimulating hormone (TSH) (human chorionic gonadotrophin – hCG) Potential for cross reaction e.g. hyperemesis Control of anterior pituitary function and biochemical testing Physiological control Static testing Dynamic testing: If you think gland is under active – try to stimulate it If you think gland is over active – try to suppress it Thyroid Axis Stimulators of TSH Pulsatile release (~9 x/24 hours) – amplitude at night Secretion stimulated by thyrotrophin releasing hormone (TRH) released into the hypohyseal portal vessels in the median eminence (TRH also stimulates prolactin release and in some circumstances growth hormone) Inhibitors of TSH Thyroid hormones directly inhibit TSH (and to a lesser extent TRH) release This can prevent the action of TRH which is basis for TRH test Dopamine and somatostatin inhibit release ?physiologically important but useful clinically for TSHomas TFT’s and Pituitary Disease TSH does not behave as it “should” with intact feedback loop Abnormal T4 concentrations without expected compensatory changes in TSH TFT’s - Lack of elevation of TSH in the presence of low T4 Indicates pituitary or hypothalamic cause of hypothyroidism – or sick euthyroid syndrome Same pattern can occur in 1st few months of treatment of thyrotoxicosis: T4 and T3 can be reduced below normal by carbimazole yet TSH remains suppressed Sick euthyroid syndrome Any severe non thyroidal illness can cause fT4 low fT3 is low or undetectable – reduced more than T4 TSH is usually normal but may be low Reverse T3 is normal or elevated Preferential production of rT3, reduced binding globulins and circulating thyroid homone binding inhibitors Clinical judgement but more common than 2º hypothyroidism TFT’s - Elevated fT4 and fT3 with failure of suppression of TSH Discordant T4 and T3 Interfering antibodies – no clinical signs Amiodarone Familial dysalbuminaemic hyperthyroxinaemia TFT’s - Elevated fT4 and fT3 with failure of suppression of TSH Other Intermittent T4 therapy Resistance to thyroid hormone* TSH secreting tumour* Acute psychiatric illness TSHoma vs hormone resistance TSHoma PRTH Clinically toxic Yes Variable Family history No Yes subunit High Normal subunit/TSH molar ratio TRH test High (>1) Normal Blunted Normal TSH response:T3 No change suppression test Peripheral action High decrease Normal Thyroid Axis Very rarely need dynamic tests TRH test usually adds little –responses vary in 2º hypothyroidism and there are easier ways to diagnose hyperthyroidism If TSHoma suspected can do TRH test and T3 suppression test (administer T3 - 80100mcg for 8-10 days - and in TSHoma TSH fails to suppress, but suppression seen in thyroid hormone resistance) Gonadal Axis Stimulators of LH/FSH Pulsatile secretion Stimulated by pulsatile secretion of gonadotrophin secreting hormone (GnRH) into the hypophyseal portal vessels GnRH release is complex and very susceptible to stress and changes to nutrition and energy homeostasis e.g. hypothalamic hypogonadotrophic hypogonadism seen in weight loss or extreme exercise Inhibitors of LH/FSH Oestradiol and progesterone inhibit LH release directly and via GnRH but in the follicular phase oestradiol becomes stimulatory inducing a surge of LH and ovulation (positive feedback) Inhibin from the ovary inhibits FSH release In the late follicular phase inhibin and oestradiol inhibit FSH release In men equally complex but more static Static testing of gonadotophins In menstruating females tests not usually needed Day 21 progesterone gives information on ovulation High prolactin can suppress gonadotrophin secretion In males if 9am testosterone is normal then gonadotrophin secretion is adequate Dynamic testing of gonadal axis Dynamic testing rarely done in adult practice GnRH test assesses reserve of LH/FSH secretion – not usually helpful Adrenal Axis Stimulators of ACTH ACTH is a single chain peptide cleaved from POMC along with MSH and endorphin (hence pigmentation in Addison’s) Secreted in pulsatile fashion in response to corticotrophin releasing hormone (CRH) – determines set point around which cortisol feedback works Circadian rhythm with superimposed effects of stress Inhibitors of ACTH Feedback from cortisol mainly directly on pituitary but also on CRH release Other adrenal androgens whose secretions are enhanced by ACTH do not have a feedback effect e.g. in congenital adrenal hyperplasia Feedback can be imitated by synthetic glucocorticoids e.g. Dexamethasone (used in suppression testing – tumorous corticotrophs less susceptible to feedback) Static testing - 9am cortisol ‘normal’ cortisol concentration does not exclude dysfunction >390 nmol/l makes deficiency unlikely (unless v sick) <100 nmol/l likely to be abnormal. Coincident ACTH can help Need further testing Salivary cortisol may become more important Dynamic testing of ACTH Adrenal Axis Underactivity ITT Synacthen test – only assesses adrenal function directly – pituitary function implied Overactivity Dexamethasone suppression test Urinary free cortisol CRH IPSS Insulin Tolerance Test Indications Assess ACTH/cortisol reserve Assess GH reserve Contraindications Ischaemic heart disease Epilepsy or unexplained blackouts Severe longstanding hypoadrenalism (liver glycogen depleted) Glycogen storage disease Hypothyroidism – untreated can give subnormal results Precautions ECG must be normal 9am cortisol must be >100 nmol/l Serum fT4 must be normal (replace 1st if low) Have resuscitation facilities, 20% glucose and IV hydrocortisone available Procedure Fast from midnight IV insulin bolus: 0.15 U/kg (0.3 U/kg for Cushing’s and acromegaly) If not hypoglycaemic at 45 mins repeat the dose Give IV glucose if severe and prolonged hypoglycaemia (>20 mins), LOC or fits – stimulus has been adequate Give lunch and sweet drink at the end of the test consider hydrocortisone Sampling Use BM sticks as guide only Lab glucose, cortisol and GH at 0,30,45,60,90 and 120mins (extend if dose repeated) Normal response (Bart’s) Lab glucose must fall to <2.2 mmol/l Serum cortisol rises by more than 170 nmol/l to at least 580 nmol/l Short Synacthen Test Cortisol response to 250mcg of tetracosactrin IV or IM (massively supraphysiological) Fasting at 9am Cortisol at 0, 30, 60 min Normal response is rise by 170 nmol/l to >580 To tell 1º vs 2º measure ACTH (or long test) 1 mcg test more sensitive to subtle adrenal dysfunction but not used routinely Synacthen test vs ITT Disadvantages Does not measure the whole axis Can be misleading after acute pituitary insult Cannot measure GH response Advantages Simpler, safer, cheaper Usually good enough in chronic situation (we tend to say >6 weeks but Synacthen test becomes abnormal after 8-12 days) Dexamethasone Suppression tests Overnight – simple but less specific – 1mg at midnight then measure cortisol at 9am: <50 nmol/l is normal (? true for modern assays) Low dose 48 hour – can be done as outpatient – 0.5mg every 6 hours: <50nmol/l is 98% sensitive High dose 48 hour – to differentiate pituitary from ectopic ACTH - 2mg every 6 hours – become redundant as performance of test is less than the pre-test likelihood of pituitary disease Dexamethasone Suppression tests Catches Rely on patient compliance if done at home Malabsorption of dexamethasone Drugs that increase hepatic clearance of dexamethasone e.g. carbamazepine, phenytoin Need to stop exogenous oestrogen for 4-6 weeks to allow cortisol binding globulin to return to basal values (assays measure total cortisol but only free is active) Growth Axis Stimulators of GH release Growth hormone releasing hormone (GHRH) stimulates synthesis/release of GH in pulsatile fashion – mostly at night Ghrelin may have a role in secretion GH exerts its effects directly and via IGF-1 production by the liver Hypoglycaemia stimulates GH release (basis of ITT for GH deficiency) Amino acids stimulate GH release (arginine can be used if ITT contraindicated) Inhibitors of GH release Somatostatin inhibits GH release Feedback from GH and IGF-1 inhibit GH release at pituitary and hypothalamic level Free fatty acids inhibit GH release Glucose inhibits GHRH and GH release (basis of GH suppression test for acromegaly) Static Testing of growth axis Random tests not helpful due to pulsatile secretion Need dynamic testing or IGF-1 GH assays Evolved from polyclonal RIA’s to 2 site monoclonal antibody non-isotopic assays with enhanced sensitivity Accurately quantify previously undetectable values Do we need age and gender dependent reference ranges ? Growth Hormone Units – a mess! Previous standard not pure & contained a number of isoforms: 22kD, 20kD and dimers/oligomers UKNEQAS showed between method variation increasing from 1994 to 1998 from 17 to 30% - most negatively biased assay reported values ½ that of most positively biased In past: UK used mU/l and US mcg/l Various conversion factors between 2 and 3 used No simple conversion factor suitable New standard EU legislation means all lab results must be traceable to a defined material (98/79/EC) Since 2001 new international standard in use (IS98/574): 22kD GH of >95% purity Now we should use mcg/l of IS98/574 We should not use mIU/l but assigned conversion factor is 3.0 IU/mg Criteria need to be looked at again Dynamic testing of GH/IGF-1 Axis Underactivity ITT Other stimulation tests e.g. glucagon, arginine Overactivity Glucose tolerance test ITT for GH deficiency Normal responses (Bart’s) GH rises to > 15 mcg/l Severe GHD needed for NICE criteria for adult GH replacement < 3 mcg/l GHD that qualifies for GH treatment in children <7 mcg/l Alternatives to ITT for GHD Glucagon Arginine Arginine plus GHRH Clonidine – in children but not adults These other tests less well validated and only used if ITT contraindicated Is ITT for GHD always needed ? Which patients do not need a GH stimulation test JCEM 87; 477-485 (2002) Pituitary hormone deficits 0 % with peak GH <2.5 mcg/l 41 1 67 2 83 3 96 4 99 If multiple pituitary axes deficient If 3 or more axes affected then test for GHD not needed – accuracy compares well with GH stimulation testing The other axes are easier to test: TSH, ACTH, gonadotrophins and vasopressin IGF-1 is not reliable for GHD IGF-1 may be normal in presence of severe GHD – it is in about a third Low IGF-1 also occurs in malnutrition, poorly controlled diabetes, oral and high dose transdermal oestrogen, hypothyroidism and hepatic insufficiency Excess – glucose tolerance test AKA growth hormone suppression test Done in same way as test for diabetes/glucose intolerance Fasting then 75g glucose load (Polycal preferred – Lucozade keeps changing! ) then sit and do nothing – no exercise, smoking, coffee ! Normal response is suppression to <0.14 mcg/l In acromegaly GH will not fall < 1 mcg/l (?still true – need to re-evaluate with new assays) False positives Failure of normal suppression but no acromegaly Diabetes mellitus Liver disease Renal disease Adolescence Anorexia nervosa False negatives can also occur New assay data Suggest we need to look again at diagnostic cut offs 25% of patients subsequently proven to have acromegaly suppressed to <1 mcg/l Prolactin Stimulators of prolactin release Released in pulsatile fashion especially at night No direct stimulatory factor Prolactin release is under tonic inhibitory control Oestrogens cause hyperplasia of lactotrophs (hence care with COC with prolactinomas) & enhance prolactin release TRH causes release of prolactin as well as TSH but this is not physiological Inhibitors of prolactin release Dopamine tonically inhibits release Impeding the hypophyseal portal circulation causes enhanced prolactin release in contrast to other pituitary hormones. Prolactin can rise to 2000 mU/l due to this ‘stalk effect’ Dopamine antagonist drugs e.g. metoclopramide, tricyclic antidepressants can stimulate prolactin release Static Testing - Prolactin If in doubt measure basal prolactin on 3 occasions Indications to measure include: Galactorrhoea Amenorrhoea/hypogonadism Infertility Pituitary mass Macroprolactin Non-bioactive prolactin: monomer of prolactin and IgG molecule with prolonged clearance rate Mass >150kDa vs 23 kDa for monomeric Accounts for 10-30% of hyperprolactinaemia Some but not all assay systems claim to detect macroprolactin but there are doubts Treat sera with polyethylene glycol to precipitate out immunoglobulins then re assay for prolactin Screening recommended for all hyperprolactinaemic sera (Clin Endo 71,466) Clinical relevance Macroprolactin is not biologically active – people with it have normal gonadal function Presence of macroprolactin usually persists If someone with gonadal dysfunction due to another cause is found to have “hyperprolactinaemia” due to macroprolactin: inappropriate dopamine agonist treatment imaging of the pituitary undertaken revealing incidentalomas (found in up to 10%) and unnecessary investigation and treatment Prevalence of macroprolactinaemia Clin Endo 71;702 (2009) 1330 hospital workers in Japan screened for hepatitis B 49 of 1330 (3.7%) had macroprolactin 15 (30.6%) of these 49 had hyperprolactinaemia – all had normal monomeric prolactin on PEG precipitation 29 of 1281 (2.26%) without macroprolactin had (true) hyperprolactinaemia Of the 44 hyperprolactinaemias, 15 had macroprolactinaemia (34%) Nobody had macroprolactinaemia and raised free prolactin All sera with macroprolactin showed complexes of prolactin and IgG – most had anti PRL Abs, with others showing a variety of prolactin complexes Total PRL-free PRL/total PRL x 100 : if >57% = macroprolactinaemia IgG bound 100% Anti PRL Abs 76% Glycosylated PRL 20% (?relevant) Of the 12 sera without antiPRL Abs Covalent disulfide bonds may be involved Suggests non covalent binding of IgG to prolactin and/or other proteins or aggregation of PRL Prolactin No dynamic tests Posterior Pituitary Vasopressin Vasopressin control Complex release in response to osmotic status and BP/circulating volume Plasma osmolality is most important: to maintain osmolality 284-295 mOsm/Kg Other factors such as drinking (suppresses VP release independent of osmolality) VP regulates water reabsorption in distal nephron via Aquaporins and regional blood flow Hypovolaemia shifts release to left→ low Na Static testing of Posterior Pituitary Paired serum and urine osmolality on rising Normal serum osmolality 280-295 mosmol/kg and concentrated urine (ratio >2:1) excludes DI In DI serum osmolality is raised and urine ratio is <2.0 (but still may be more than serum in mild cases) Most need water deprivation test Dynamic testing: Water deprivation test Diagnosis of diabetes insipidus Differential diagnosis of thirst polyuria and nocturia Precautions Need to watch for dehydration Thyroid function and adrenal reserve must be normal or replaced Close rapid liaison with lab is vital – results are needed quickly – ensure lab know the test is going on Procedure Allow fluids until 07.30 but no tea, coffee or smoking No food or fluid from 07.30 Weigh patient and work out 97% of weight Directly supervise patient to avoid cheating 8 hours water deprivation unless stopping early Weight Weigh basally and at 4,6,7 and 8 hours If >3% weight lost send urgent serum osmolality if >300 mosmol/kg give DDAVP and allow to drink If <300 mosmol/kg patient was probably fluid overloaded at the start of the test Biochemical monitoring Hourly urine vols and osmolality Hourly serum osmolality Record results on proforma Give 2mcg desmopressin IM: If weight falls >3% and serum osmolality >300 If serum osmolality >300 and urine osmolality <600 Then measure urine vols and osmolalities for further 2-4 hours (allow to eat and drink) Interpretation If urine osmolality <600 and serum osmolality > 300 after 8 hours of fluid deprivation then diagnosis is DI. Assuming urine concentrates to >600 after DDAVP administration diagnosis is cranial DI. If this does not occur diagnosis is nephrogenic DI. Urine osmolality >600 in context of normal serum osmolality after 8 hours excludes DI and no need for DDAVP administration. Consider psychogenic polydipsia if basal serum osmolality is <260 in presence of low urine osmolality. Prolonged WDT (Miller and Moses) For mild DI where serum fails to concentrate to >300 after 8 hours water deprivation Unless symptoms very mild do standard WDT 1st Patient nil by mouth from 6pm the night before – so patient starts more dehydrated Otherwise interpretation the same Excess - SIADH SIADH – syndrome of inappropriate ADH secretion 1st described 1967 Essential criteria: 1. Plasma osmolality <270 mOsm/kg 2. Inappropriate urinary conc (>100 mOsm/kg) 3. Clinical euvoloaemia 4. High urinary Na (>40 mmol/l) with normal salt and water intake 5. Hypothyroidism & glucocorticoid deficiency excluded Not fully understood 4 types depending on pattern of ADH release Why do patients continue to drink despite plasma osmolality below thirst threshold ? Hyponatraemia is limited by ‘escape from antidiuresis’: urine flow rises and urine osmolality falls and sodium stabilises in hyponatraemic range Causes of SIADH 1. 2. 3. 4. Tumours Pulmonary disease CNS disease Drugs: Phenothiazines, TCA’s, chlorpropamide, ecstasy, carbamazepine, cyclophosphamide, SSRI’s, others Approach to the hyponatraemic patient Identify clinical signs of underlying disease e.g. Addison’s Identify ECF status Measure urinary sodium and osmolality Check TFT’s (and cortisol +/- synacthen) CXR – for fluid status and underlying disease Clinical Scenario’s Investigation of suspected Cushing’s Clinical features Obesity/wt gain Facial plethora Round face Thin skin Hypertension Hirsutism Easy bruising Glucose intolerance Proportion (%) 95 90 90 85 75 75 65 60 Terminology Cushing’s syndrome – the biochemical syndrome of cortisol excess Cushing’s disease – the specific cause of the Cushing’s syndrome is a pituitary adenoma How picked up Can be florid Can be subtle – probably many are missed in hypertension, sleep apnoea and diabetes clinics Needs to be ruled out before bariatric surgery Process 1. 2. 3. Prove cortisol excess Decide if ACTH dependent or ACTH independent If ACTH dependent – decide if pituitary or ectopic ACTH Bear in mind Cushing’s disease Ectopic ACTH Unknown ACTH Adrenal adenoma Adrenal Ca Macronod hyperpl Pigmented nod McCune-Albright Proportion (%) 70 10 5 10 5 <2 <2 <2 Female:male 3.5:1 1:1 5:1 4:1 1:1 1:1 1:1 1:1 Diagnosing hypercortisolaemia LDDST – 3-8% with Cushing’s suppress (if high suspicion do DS-CRH test- 15 mins post CRH cortisol >38 nmol/l=CS). False positives more common Overnight DST – less specific with more false positives 24 hour UFC – need repeated tests as single measurements have low sensitivity. Collections often incomplete Midnight cortisol – plasma or salivary – look for loss of diurnal variation. Midnight plasma cortisol excludes Cushing’s but needs admission. Salivary cortisol : renewed interest as sens and spec 95-98% and is surrogate for plasma free cortisol Establish the cause Measure ACTH: < 5 pg/ml = ACTH independent >15 pg/ml = ACTH dependent ACTH Independent Image adrenal glands ACTH dependent Remember 70%+ will be pituitary – more in females Don’t rely on imaging to tell pituitary from ectopic Pituitary MRI often normal in Cushing’s Disease (up to 40%) – adenoma’s small Dynamic tests High dose DST: 80% of patients with Cushing’s show cortisol suppression of > 50%. Adds little and not needed if >30% suppression on low dose CRH test – measure cortisol and ACTH response to ovine or human CRH. In Cushing’s disease CRH responsiveness persists (20% rise) unlike ectopic. Responses variable but sensitivity 86-93% for Cushing’s disease If ACTH dependent Cushing’s proven with typical responses on DST and CRH and 6mm lesion or more on MRI then reasonable to operate Otherwise more info needed Inferior petrosal sinus sampling Inferior petrosal sinus sampling Sample gradient of ACTH from the pituitary to the periphery Site cannulae under venographic screening Cushing’s disease: Basal central:peripheral ratio of 2:1 CRH stimulated ratio of 3:1 ‘Gold standard’ but not perfect: false positives and negatives (<10%) described Only 70% accurate for lateralisation in adults Overall Measure ACTH < 5pg/ml >15 pg/ml Adrenal imaging PituitaryMRI +/- CRH +/- High dose DST Adenoma surgery No adenoma IPPS Clinical Case – Mr MJ Age 57 Seen in diabetes clinic Hypertensive and obese (BMI >50) Somewhat Cushingoid in appearance but no striae Initial screen 24 hour UFC: 1189 nmol/d 855 nmol/d Reference range 40-305 nmol/d Prolactin 133, testo 2.9, IGF -1 20.4, TSH 1.7 fT4 15 Confirming Cushing’s Syndrome – 48 hour low dose DST Cortisol: 515 nmol/l → 376 nmol/l (27% reduction) Confirming ACTH dependence Basal ACTH 63 ng/l Confirming pituitary source – high dose DST 519 nmol/l → 75 nmol/l Confirming pituitary source – CRH test 0 15 30 45 60 90 120 Corti 361 sol 442 565 559 524 476 429 ACT H 190 199 163 133 82 66 68 Imaging Showed small adenoma on CT then MRI In view of evidence (and patients obesity) IPPS not done Transphenoidal Surgery Adenoma with ACTH staining removed 30 kg weight loss Diabetes resolved BP easier to control Initial synacthen test abnormal – subsequently normalised so now off hydrocortisone replacement and remains well with no signs of recurrence Treatment – transsphenoidal surgery Treatment – transsphenoidal surgery up to 90% remission rate in microadenomas, less if no obvious tumour or large tumour Patients require steroid cover afterwards – if they don’t they are not cured ! Complication rate 14% with mortality of 2% Adrenalectomy Cures all forms of ACTH independent Cushing’s syndrome Bilateral adrenalectomy for ACTH dependent Cushing’s but risk of Nelson’s syndrome Radiotherapy Primary radiotherapy: long term remission of 37% Usually used 2nd line after surgery: 88% remission but can take 5 years – usually results in other pituitary hormone deficits (GH>hypogonad>hypothyroid>ACTH) Medical therapy -metyrapone inhibits 11 hydroxylase so blocks: 11deoxycortisolcortisol 11 deoxycortisol levels rise: in some assays 11 deoxycortisol cross reacts with cortisol. Can result in unnecessary increase in treatment ACTH levels rise but do not usually overcome block Rise in adrenal androgens causes hirsutism Medical therapy - ketoconazole Antifungal agent Inhibits several enzymes in steroid synthesis pathway Also reduces adrenal androgens so no hirsutism (and cholesterol) Can be hepatotoxic Interacts with simvastatin Anaesthetic agent etomidate works similarly Monitoring treatment Cortisol day curves (mean cortisol between 150 and 300 nmol/l corresponds with a normal cortisol production rate) – beware cross reactivity with 11 deoxycortisol if on metyrapone 24 hour UFC’s Acromegaly Diagnosis GH suppression test IGF-1 Treatment Surgery- cure up to 90% micro, <60% macro Radiotherapy – can take several years to work Dopamine agonists –effective in up to 30% Somatostatin analogues- effective in 66%. Can be used pre-op to shrink & soften – can shrink by 40% Pegvisomant - GH receptor antagonist Monitoring IGF-1: in ref range GH suppression test: nadir <1 mcg/l GH day curve: mean <2.5 mcg/l normalises mortality (but used old polyclonal immunoassays) Controversial ! What if there is discordance ? Can’t monitor GH if on pegvisomant Issues Probably need age, gender and BMI specific GH cut offs (GH levels are lower with increasing age and BMI. Females have higher GH nadir) Others things affect GH suppression e.g renal failure, diabetes Other things affect IGF-1 levels e.g. malnutrition, liver disease, hypothyroidism Changes in IGF-1 normative data Discordance between GHST and IGF-1 Most commonly normal IGF-1 but high GH Repeat tests after 3 months – usually doesn’t help Somatostain analogues have less effect on GHST than IGF-1 Most people would follow IGF-1 result but watch closely for recurrence if GH suppression is abnormal (evidence of increased recurrence rate) Clinical Case 30 year old female Noticed blurred vision Optican found visual field defect – confirmed as bitemporal by ophthalmologist 6 months amennorrhoea On questioning – increased shoe size and had to change wedding ring twice. Sweating more. On examination Clinically acromegalic with prominent nasal bridge and jaw Large hands Large tongue with indentation due to teeth Initial results IGF1 Prolactin Cortisol SST fT4 TSH Oestradiol 105nmol/l (13-50) 1154 miu/l (<500) 353 nmol/l 367→535→646 nmol/l 9 pmol/l (9-23) 1.77 miu/l (0.25-5) 160 pmol/l (100-750) GH Suppression Test Basal 40 min 70 min 100 min 130 min GH 11.4 (mcg/l) 10.8 10.1 9.7 9.9 Glucose 4.7 (mM) 5.8 5.7 5.7 5.2 Imaging Large pituitary tumour touching the chiasm Treatment Plan Not curable with surgery Pre-op octreotide (to shrink tumour and soften it) then surgery Followed up with octreotide and radiotherapy May need pegvisomant Prolactinoma Commonest functioning pituitary tumour Present earlier and also more common in women – die to amenorrhoea In men may just present with local pressure effects Many causes of high prolactin Prolactinoma Drugs: DA antagonists, neuroleptics, antidepressants Non functioning tumour (<2000) Pregnancy/lactation Hypothyroidism Renal failure PCOS Lab issues Remember macroprolactin – screen all samples Prolactin levels can be very high (>100,000) so if clinical suspicion high but prolactin levels normal look for hook effect by diluting samples (however modern assays can measure very high concentrations) Features - hormonal Galactorrhoea Menstrual disturbance Reduced libido/erectile dysfunction Osteoporosis (long term) Features - mass Headaches Visual field loss Hypopituitarism Cranial nerve palsies CSF leak (rare) If prolactin high but on drug known to increase prolactin Dilemma Consider if timing fits Can drug be withdrawn or changed ? Just a few days is enough for oral medications Often end up doing MRI but incidentalomas are common Prolactinoma If PRL >2000 very likely prolactinoma, if >5000 definitely so If unsure if functioning or not can treat and rescan Prolactin levels will fall with medical therapy anyway – more so if not prolactinoma Medical management – Dopamine agonists Bromocriptine Cabergoline Quinagolide – being used more as no known risk of heart valve fibrosis Clinical case – Mr JF 42 year old man with erectile dysfunction No other symptoms 10 year old child Prolactin 23,000 with negative macroprolactin screen Testosterone 2.0 nmol/l, LH <0.1 U/L fT4 23 TSH 2.43, SST normal, IGF-1 18.1 MRI: macroadenoma but away from chiasm On Cabergoline Prolactin now 686 Testosterone no better Started on testosterone replacement – gel then Nebido and erectile problems resolved Pituitary lesion smaller fT4 11 TSH 2.86 so started on thyroxine in case secondary hypothyroidism Pituitary Apoplexy Pituitary Apoplexy Acute infarction or haemorrhage of the pituitary Usually an adenoma is present Acute headache (retro-orbital), visual disturbance, altered mental function, cranial nerve palsies & endocrine dysfunction Can occur post partum in nontumorous glands – Sheehan’s Syndrome Management Endocrine emergency Send off baseline bloods – cortisol, TFT’s, IGF1, LH/FSH, testo/oestradiol, prolactin Urgent steroid replacement with high dose hydrocortisone or dexamethasone Urgent discussion with neurosurgeons – if compression of chiasm or cranial nerve palsy surgery is indicated Learning Objectives Describe pituitary anatomy and endocrine physiology Describe methods for assessing pituitary function using static and dynamic testing Describe the new standard for the measurement of growth hormone & its effects on clinical criteria Be are of the importance of screening for macroprolactin