* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Document
Psychoneuroimmunology wikipedia , lookup
Globalization and disease wikipedia , lookup
Adaptive immune system wikipedia , lookup
DNA vaccination wikipedia , lookup
Cancer immunotherapy wikipedia , lookup
Molecular mimicry wikipedia , lookup
Adoptive cell transfer wikipedia , lookup
Polyclonal B cell response wikipedia , lookup
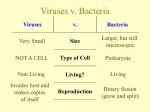
Mohammed El-Khateeb HIV/AIDS DVL-3 April 5th 2015 Overview • History • Organism • Epidemiology • Transmission • Disease in Humans • Disease in Animals • Prevention and Control HIV and AIDS History of an infectious agent In Los Angeles 1967-1978: only two cases of Pneumocystis carinii pneumonia 1979 - 5 cases of Pneumocystis carinii pneumonia All Homosexual Dot-like intracystic bodies of Pneumocystis carinii in lung Cytologic preparation from a bronchoalveolar lavage – Giemsa stain Kaposi’s sarcoma rare (immunocompromization) , Elderly 1981 - 26 cases of Kaposi’s sarcoma • • • • Young Male San Francisco and New York All Homosexuals HIV and AIDS •Two rare diseases in the gay community linked to •IMMUNOSUPPRESSION •OPPORTUNISTIC INFECTIONS •Lymphadenopathy •Hodgkin’s Lymphoma Little in common but: • Young • White • Male • Large towns • Homosexual community • Gay-Related Immune Deficiency • Acquired Immune Deficiency Syndrome (AIDS) But not all gay men got the disease Distinguishing characteristics Clusters of infected men Apparent concentration within sexually interactive groups High numbers of sex partners Different ways of getting a similar syndrome [Blood transfusions, Intravenous drug use, Hemophilia (clotting factor)])) Suggests an infectious agent A retrovirus causes AIDS In May 1983: doctors at the Institute Pasteur in France reported that they had isolated a new virus, which they suggested might be the cause of AIDS. BarreSinoussi F. … and Montagnier L. (1983), 'Isolation of a T-Lymphotropic retrovirus froma patient at risk for Acquired Immune Deficiency Syndrome (AIDS)', Science, May 20 In May 1983: doctors at the NIH in the US reported that a retrovirus virus related to Human T cell Leukemia virus was present in patients with AS. Gelman EP … and Gallo RC. (1983), “Proviral DNA of a retrovirus, human T-cell leukemia virus, in two patients with’AIDS, Science, May 20 January 1985 It becomes clear that LAV and HTLV-III are the same virus and The FDA licenses the first blood test for AIDS. (HIV-1) 1986: HIV-2 discovered in antigenicaly distinct virus endemic in West Africa. Lentivirus All Retroviruses contain gag, pol, pro* and env Lentiviruses are more complex: • Two regulatory genes: tat and rev • Accessory genes: • HIV-1 nef, vif, vpr, vpu • HIV-2 and SIV lack vpu and have vpx *pro is sometimes contained within pol (HIV) Diagram of Immunodeficiency Virus (HIV) Retroviruses have stripped down genomes So they make good use of what they have How does HIV infect new people CD4 and CXCR4 CD4 & CCR5 found on macrophages Viral Replication Transmission • HIV can be transmitted via the exchange of a variety of body fluids from infected individuals, such as • Individuals cannot become infected through ordinary day-to-day contact such as kissing, hugging, shaking hands, or sharing personal objects, food or water. How is HIV contracted? DANGER • • • • • • • • • Health Care Setting Tattoos / Piercings Blood Transfusions Blood Products Mother to Child Oral Sex Vaginal Sex Anal Sex Injecting Drugs ALL CLEAR AHEAD Sneezing / Coughing Sharing Glasses Showers / Pools Protected Sex Insects Kissing* HIV in different body fluids Blood 18,000 Semen 11,000 Vaginal Fluid 7,000 Breast Milk 4,000 Saliva 1 Average number of HIV particles in 1 ml of these body fluids Immune Responses to Infection Virion Antigen Presenting Cell (APC, Phagocyte) Immune Responses to Infection Virion CD8+ T Cell Killer T Cells Antigen Presenting Cell (APC, Phagocyte) Kill Infected Cells Immune Responses to Infection Virion CD8+ T Cell Killer T Cells Kill Infected Cells Antigen Presenting Cell (APC, Phagocyte) B Cell Antibodies Coat Pathogen Immune Responses to Infection Virion CD8+ T Cell Killer T Cells CD4+ T Cell T Helper Cells Antigen Presenting Cell (APC, Phagocyte) B Cell Early Events in HIV Infections Latent vs. active infection In latent infection- retroviral genome is present but is not transcribing viral genome or mRNA for structural proteins. 21 HIV - Life History Latency – Cellular – The problem of memory T4 cells Only activated T4 cells can replicate virus Most infected T4 cells are rapidly lyzed but are replaced Some T4 cells revert to resting state as memory cells which are long-lived Memory T4 cells cannot replicate the virus unless they become activated Clinical Latency HIV infection is not manifested as disease for years During apparent clinical latency, virus is being replicated and cleared 22 Stages of HIV Infection Course of HIV Infection P24 antigen Anti-HIV IgM Window: 3-5 days ‘Sensitive’ Combo assays 24 Mononucleosis-like, cold or flu-like symptoms may occur 6 to 12 weeks after infection. Lymphadenopathy Fever Rash Headache Fatigue Diarrhea Sore throat Neurologic manifestations. no symptoms may be present HIV and AIDS The cellular and immunological picture The course of the disease 1. Acute Infection • • • • • • High virus titer Mild symptoms Fall in CD4+ cells but recovers Rise in CD8+ cells but recovers A high virus titer (up to 10 million viruses per ml blood) Macrophages infected 26 HIV and AIDS 2. A strong immune response Virus almost disappears from circulation • Good cytoxic T cell response • Soluble antibodies appear later against both surface and internal proteins • Most virus at this stage comes from recently activated (dividing) and infected CD4+ cells • CD4+ cell production compensates for loss due to lysis of cells by virus production and destruction of infected cells by CTLs 27 HIV and AIDS 3. A latent state Latency of virus and of symptoms • Virus persists in extra-vascular tissues • Lymph node dendritic cells • Resting CD4+ memory cells (last a very long time - a very stable population of cells) carry provirus 28 HIV and AIDS 4. The beginning of disease •Massive loss of CD4+ cells • CD4+ cells are the targets of the virus • Cells that proliferate to respond to the virus are killed by it • Dendritic cells present antigen and virus to CD4 cells • Epitope variation allows more and more HIV to escape from immune response just as response wanes • Apoptosis of CD4+ cells • HIV patients with high T4 cell counts do not develop AIDS 29 HIV and AIDS 5. Advanced disease - AIDS CD8+ cells destroy more CD4+ cells • CD4 cell loss means virus and infected cells no longer controlled • As CD4+ cells fall below 200 per cu mm virus titer rises rapidly and remaining immune response collapses • CD8+ cell number collapses • Opportunistic infections • Death in ~2 years without intervention 30 HIV and AIDS 10 billion HIV particles per day Virus half life 5.7 hours 10 -100 million virions per ml blood (set point) Small minority of T4 cells are infected Virus found in lymph nodes 31 Opportunistic Infections DIAGNOSIS Diversity Within the Individual Homogeneous new infection Replicates ~ 24hrs Produces 1010 new virions a day Average 1 mutation per replicated genome Rapidly develop a “quasispecies” HIV-1 Diversity Worldwide HIV-1 group M: - 9 subtypes (>30% difference) several circulating recombinant forms Subtype A B C D F, G, H, J. K CRF01_AE CRF02_AG CRF03_AB other Hemelaar et al. 2004. WHO/UNAIDS. Diagnostic Assay Requirements MUST DETECT ALL SUBTYPES Antigen Detection Antibody Detection PCR Quantitation Seroconversion Resistance mutants Different assays vary in their ability to detect various HIV subtypes Laboratory Diagnosis Antigen / antibody detection ELISA, serum HIV-1 & -2 antibodies, HIV-1 CA (p24) antigen Screening of blood donors Western Blotting PCR Viral RNA or DNA provirus Blood or tissue specimens Quantitative PCR (viral load): to determine disease stage and treatment follow up. Classification of Antiretroviral medicines • NRTI (Nucleoside Reverse Transcriptase Inhibitors) • NtRTI (Nucleotide Reverse Transcriptase Inhibitors) • NNRTI ( Non-Nucleoside Reverse Transcriptase Inhibitors) • PI (Protease Inhibitors) • Entry Inhibitors • Integrase Inhibitors Reverse Transcription Inhibitors Protease Blockers X Highly Active Anti-Retroviral Therapy Vaccines • Preventative – Subunit Vaccines – Recombinant Vector Vaccines – DNA Vaccines • Therapeutic VACCINE Vaccine Design DNA How Does this Vaccine Work? A few HIV genes are inserted into a backbone of DNA known as plasmid The vaccine is injected into muscle of the recipient where the HIV genes are expressed into proteins. The viral proteins are degraded into small peptide fragments, which are then presented by molecules on the cell surface. T cells recognizing these molecules generate an immune response. Live Vectors: (Viral and Bacterial) No DNA vaccines have yet been approved for use in humans by the FDA. FDA guidance on DNA vaccines can be found on FDA'sConsiderations for Plasmid DNA Vaccines for Infectious Disease Indications The HIV or SIV genes are inserted into the genomes of live, infectious, but non- The development of viral vectors has been disease-causing forms of viruses (e.g., adenovirus) or bacteria (e.g., Bacille robust, with a few entering Phase III Calmette-Guerin (BCG). trials. These vectors shuttle “foreign” genes along with their own into cells. Only a few bacterial vectors are under development in small and large HIV proteins generated from these recombinant genes inside the cell are either animal models, and some Phase I secreted or displayed on the cell surface and presented to the immune trials. The complex nature of system. bacteria hampers the development of bacterial vector systems. Viral Chemically synthesized pieces of HIV peptides or proteins that elicit strong T and B cell responses. Proteins or Viral Peptides Virus-like Particles (VLPs) Issues for HIV Vaccine Empty, non-infectious shells of the HIV envelope protein; they mimic the outer coat of the virus but lack a genome inside and cannot reproduce. Because VLPs resemble the virus, they can induce high titers of neutralizing antibodies to protect against viral challenge. Peptide-based preparations require the addition of an adjuvant to enhance immunogenicity. At present, alum is the only adjuvant authorized by FDA for general medical use, however many products are being tested, and some are in clinical trials. VLPs represent an exciting new strategy for HIV vaccines but it has been difficult to make them reproducibly. Long-term control of HIV by CCR5 Δ32/Δ32 stem cell transplantation Chemotherapy (x4) Total-body irradiation (x2) CCR5 - off ART no viral rebound scBMT (X2) CCR5+ CCR5 - HIV-1+ AML HIV-1– ? Donor Number of people living with HIV Total Adults Women Children (<15 years) 35.0 million [33.2 million – 37.2 million] 31.8 million [30.1 million – 33.7 million] 16.0 million [15.2 million – 16.9 million] 3.2 million [2.9 million – 3.5 million] 2014 epidemiology core slides Global summary of the AIDS epidemic2013 Total 2.1 million [1.9 million – 2.4 million] People newly Adults 1.9 million [1.7 million – 2.1 million] infected Children (<15 years) 240 000 [210 000 – 280 000] with HIV in 2013 AIDS deaths in Total 1.5 million [1.4 million – 1.7 million] 2013 Adults 1.3 million [1.2 million – 1.5 million] Children (<15 years) 190 000 [170 000 – 220 000] 45 Adults and children estimated to be living with HIV2013 Eastern Europe & Central Asia North America and Western and Central Europe 2.3 million 1.1 million [980 000– 1.3 million] [2.0 million – 3.0 million] Middle East & North Africa Caribbean 250 000 [230 000 – 280 000] Latin America 1.6 million [1.4 million – 2.1 million] 230 000 [160 000 – 330 000] Asia and the Pacific 4.8 million Sub-Saharan Africa [4.1 million – 5.5 million] 24.7 million [23.5 million – 26.1 million] Global total: 35.0 million Sub-Saharan Africa: 24.7 million Eastern and Southern Africa (ESA) Region: 18.5 million 46 Estimated number of adults and children newly infected with HIV2013 Eastern Europe & Central Asia 110 000 North America and Western and Central Europe [86 000 – 130 000] 88 000 [44 000 – 160 000] Middle East & North Africa Caribbean 12 000 [9400 – 14 000] Latin America 94 000 [71 000 – 170 000] 25 000 [14 000 – 41 000] Asia and the Pacific 350 000 Sub-Saharan Africa [250 000 – 510 000] 1.5 million [1.3 million – 1.6 million] Total: 2.1 million Sub-Saharan Africa: 1.5 million Eastern and Southern Africa (ESA) Region: 1.1 million 47 Estimated adult and child deaths from AIDS2013 Eastern Europe & Central Asia 53 000 North America and Western and Central Europe [43 000 – 69 000] 27 000 [23 000 – 34 000] Middle East & North Africa Caribbean 11 000 [8300 – 14 000] Latin America 47 000 [39 000 – 75 000] 15 000 [10 000 – 21 000] Asia and the Pacific 250 000 Sub-Saharan Africa [210 000– 290 000] 1.1 million [1.0 million – 1.3 million] Global total: 1.5 million Sub-Saharan Africa: 1.1 million Eastern and Southern Africa (ESA) Region: 730,00 48 12,9 million people on ART at the end of 2013 globally. 11,7 in low-and middle income countries - 2 million more than at the end of 2012 Actual and projected numbers of people receiving antiretroviral therapy in low-and middle-income countries, and by WHO Region, 2003–2015 Source: 2014 Global AIDS Response Progress Reporting (WHO/UNICEF/UNAIDS). 2013 المفرق المفرق المفرق المفرق المفرق المفرق 66 66 66 اربد اربد اربد اربد 41 39 41 41 عجلون عجلون عجلون عجلون عجلون عجلون 11 2111 جرش جرش جرش جرش 2 222 الزرقاء الزرقاء الزرقاء الزرقاء 31عمان 29 29 عمان 29 عمان 29 عمان البلقاء البلقاء البلقاء البلقاء 149 149 149 10 183 11 10 10 مادبا مادبا مادبا مادبا 3333 أردني غير اردني المجموع الكرك الكرك الكرك 222 283 739 1022 3خارج االردن 3غير معروف معان معان 11 الطفيلة الطفيلة 22 العقبة 1 This NAP Webpage (www.moh.gov.jo) 51 Dream ZERO NEW INFECTION ZERO DEATH ZERO DISCRIMINATION Promise and Accountability 52 Important to mobilize all sectors for HIV/AIDS response: leaving no one behind Ambition will be required to get ahead of the epidemic and save lives Thank you ! 53