* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download St. Michael`s/U of T Presentation Template
Nicotinic agonist wikipedia , lookup
Pharmacogenomics wikipedia , lookup
NK1 receptor antagonist wikipedia , lookup
Drug interaction wikipedia , lookup
Toxicodynamics wikipedia , lookup
Discovery and development of antiandrogens wikipedia , lookup
Drug discovery wikipedia , lookup
Neuropsychopharmacology wikipedia , lookup
Pharmacokinetics wikipedia , lookup
Neuropharmacology wikipedia , lookup
Psychopharmacology wikipedia , lookup
Drug design wikipedia , lookup
Theralizumab wikipedia , lookup
Dydrogesterone wikipedia , lookup
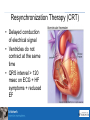
New Therapies Heather Kertland, PharmD New Agents Outline • Eplerenone • Ultrafiltration • CRT Rales Study Population NYHA Class III and IV heart failure and LVEF≤35% Comparator spironolactone target dose 50 mg/day vs. placebo Outcome n = 1,663; mean follow-up = 24 months; mean LVEF 25.6%; mean dose = 26 mg/day; All-cause mortality: spironolactone was associated with a 30% RRR compared to placebo (34.5% vs. 46%, p<0.001) ARR = 11%; NNT = 9 Safety Hyperkalemia ≥ 6.0 μmol/L: 2% spironolactone vs. 1% placebo (p=0.42) Gynecomastia in men: 9% spironolactone vs. 1% placebo New Engl J Med 1999 Eplerenone (Inspra) • A chemical derivative of spironolactone, • enhanced selective binding to the mineralocorticoid receptor • minimizing binding to progesterone and androgen receptors Ephesus trial Population 3 to 14 days post-MI with either symptoms of HF or diabetes without HF symptoms; NYHA classes I-IV; LVEF≤40% Comparator eplerenone target dose 50 mg/day vs. placebo Outcome n = 6,642; mean follow-up 16 months; mean LVEF 33%; mean dose = 43.5 mg/day; All-cause mortality: eplerenone demonstrated a 15% RRR compared to placebo (14.4% vs. 16.7%; p=0.008) ARR = 2.3%; NNT = 43 Composite endpoint of death from CV causes or first hospitalization from a CV event: eplerenone demonstrated a 13% RRR compared to placebo (26.7% vs. 30.0%, p=0.002) ARR = 3.3%; NNT = 30 New Engl J Med 2003;348: Ephesus Safety Hyperkalemia ≥ 6.0 μmol/L: 5.5% eplerenone vs. 3.9% placebo Gynecomastia in men: 0.5% eplerenone vs. 0.6% placebo Other DI: substrate for CYP3A4 (concomitant use of strong CYP3A4 inhibitors is contraindicated) Hyperkalemia New Engl J Med 2004;351:542-51 Rx info • Monitoring K+ – 48 hours post 1st dose – 1,4 and 5 weeks – 1 week after any dose change • Coverage – Common Drug Review (CDR) – do not cover – ODB • Exceptional Access • Experience AMI, have evidence of heart failure and left ventricular systolic dysfunction (EF <40%) and tried spironolactone but experienced severe symptomatic (painful) gynecomastia. Ultrafiltration • Pts not responding to diuretics • Clinic/inpatient setting • Results 2 – 3 kg greater weight loss than diuretics, no effect on dyspnea • No adverse effects on Serum Cr • Anticoagulation is required SMH experience Endpoint N=8 Age 66.1 yrs Duration of UF 36 hrs (15 – 65 hrs) UF rate 50 – 250 mL/hr Average weight loss 7.5 kg SMH Experience 350 a b c d e f g i j 300 250 200 150 100 50 0 baseline Cr Post UF Cr Summary • UF is an alternative to diuretics for removing fluid • Well tolerated with respect to renal function, although can remove fluid too quickly/too much • No known effects on drug concentrations determined to date Resynchronization Therapy (CRT) • Delayed conduction of electrical signal • Ventricles do not contract at the same time • QRS interval > 120 msec on ECG + HF symptoms + reduced EF New Engl J Med 2010 Summary • • • • Indication is heart failure not bradycardia Can have pacing capabilities Can be combined with ICD Impact on drug therapy – Implantation – holding of anticoagulation – Post – improvement in symptoms – decrease in furosemide