* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Chemistry PowerPoint
Chemical bond wikipedia , lookup
Water splitting wikipedia , lookup
Electronegativity wikipedia , lookup
Physical organic chemistry wikipedia , lookup
Safety data sheet wikipedia , lookup
Electrochemistry wikipedia , lookup
Nuclear transmutation wikipedia , lookup
Chemical reaction wikipedia , lookup
Biochemistry wikipedia , lookup
Freshwater environmental quality parameters wikipedia , lookup
Electron configuration wikipedia , lookup
Artificial photosynthesis wikipedia , lookup
Electrolysis of water wikipedia , lookup
Abundance of the chemical elements wikipedia , lookup
Gas chromatography–mass spectrometry wikipedia , lookup
Chemical thermodynamics wikipedia , lookup
Periodic table wikipedia , lookup
Isotopic labeling wikipedia , lookup
Evolution of metal ions in biological systems wikipedia , lookup
Metalloprotein wikipedia , lookup
History of molecular theory wikipedia , lookup
Atomic nucleus wikipedia , lookup
Chemical element wikipedia , lookup
Stoichiometry wikipedia , lookup
Extended periodic table wikipedia , lookup
History of chemistry wikipedia , lookup
IUPAC nomenclature of inorganic chemistry 2005 wikipedia , lookup
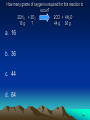
LESSON 25 IDENTIFYING CHEMICALS 1 Which of the following is a definition of an element? a. An element is a natural substance that can be used to build matter b. An element is a substance that cannot be easily changed into another substance. c. An element is a substance that can be found only in mixtures d. An element is a substance that can be made only by humans 2 Which of the following is a synthetic chemical? a. Water b. Salt c. Oxygen d. Pesticides 3 Which of the following is a synthetic element? a. C b. Ca c. Al d. Pu 4 Which of the following is an element? a. NaCl b. HF c. He d. KBr 5 Which of the following is a compound, not a mixture? a. Air b. Container of sand, salt and charcoal c. Trail mix d. Table salt 6 Which of the following represents a mixture? a. A piece of copper wire b. The oxygen gas in a pressurized tank c. A slice of mushroom pizza d. A lead fishing weight 7 LESSON 26 ELEMENTS FORM COMPOUNDS 8 Which of the following can be broken down into simpler substances? a. H b. Na c. Cl d. NaCl 9 Which of the following shows a chemical formula with 4 oxygen atoms? a. NaOH b. C6H12O6 c. H2O d. KMnO4 10 The smallest unit of an element that has all the properties of the element is called what? a. Atom b. Element c. Compound d. Cell 11 Which atomic particle is correctly matched with its charge? a. Proton; negative b. Electron; positive c. Neutron; negative d. Electron; negative 12 Which atomic particle is correctly matched with its location? a. Proton; orbits the nucleus b. Neutron; orbits the nucleus c. Electron; in the nucleus d. Proton; in the nucleus 13 Which of the following contain the greatest number of different elements? a. NaCl b. H2O c. CH4 d. C6H12O6 14 Which of the following shows a ration of N to O atoms of 1:2? a. NO b. NO3 c. NO2 d. CO2 15 Which of the following is correctly arranged in going from simplest to most complex? a. Compound element a. Atom element b. Atom compound c. Element atom atom compound element compound 16 LESSON 27 THE PERIODIC TABLE 17 An atom has 6 protons, 6 electrons and 8 neutrons. What is its atomic mass? a. 6 b. 8 c. 12 d. 14 18 A charged atom has 8 protons, 10 electrons and 9 neutrons. What is its atomic number? a. 8 b. 9 c. 10 d. 17 19 Which of the following is the basis for arranging the elements in the periodic table? a. Alphabetical order b. Masses of atoms c. Number of protons d. Date of discovery 20 How many elements are listed in the periodic table? a. 60 b. 72 c. 92 d. More than 100 21 What is the atomic number of He? a. 1 b. 2 c. 4 d. 18 22 Which element has similar chemical and physical properties to Cl? a. O b. Ne c. S d. Br 23 Which element does NOT have similar properties to K? a. H b. Na c. Fr d. Ca 24 Which of the following is a metalloid? a. Na b. Zn c. Cl d. As 25 Which of the following is a metal? a. O b. Si c. Br d. K 26 Which of the following is a nonmetal? a. Li b. Ca c. B d. N 27 All elements in Group 18 are what? a. Liquids b. Solids c. Metalloids d. Gases 28 Which element is found in period 2, group 1? a. Na b. Mg c. Li d. Be 29 Of the following, which is listed last on the periodic table? a. Atomic # 7 b. Oxygen c. Atomic # 2 d. Helium 30 LESSON 28 PROPERTIES OF MATTER 31 Which of the following is an example of a chemical property? a. Density b. Magnetism c. Electrical conductivity d. Chemical reactivity 32 Which of the following is malleable? a. Sulfer (S) b. Aluminum (Al) c. Bromine (Br) d. Oxygen (O) 33 Which of the following is NOT ductile? a. Iron (Fe) b. Lead (Pb) c. Tin (Sn) d. Neon (Ne) 34 Why is copper used in electrical wiring? a. Is has good electrical conductivity b. It is ductile c. It is a poor insulator d. All of the above 35 The formula for density is what? a. D = V/M (Volume divided by Mass) b. D = Mass x Volume c. D = M/V (Mass divided by Volume) d. D= Length x Width x Height 36 A sample of lead has a mass of 33 grams and a volume of 3 cubic centimeters. What is the density of lead? a. 99 grams/cubic centimeters b. 33 grams/cubic centimeters c. 11 grams/cubic centimeters d. 3 grams/cubic centimeters 37 LESSON 29 IDENTIFYING SUBSTANCES 38 Melting point is a physical property. What is melting point? a. The temperature at which the liquid turns to a solid b. The temperature at which the solid turns to a liquid c. The temperature at which the substance forms a gas d. The amount of heat the substance absorbs 39 LESSON 30 MEASURING PHYSICAL AND CHEMICAL CHANGES 40 Which of the following is a chemical change only? a. Tearing paper b. Sharpening a pencil c. Burning paper and pencil d. Writing with a pencil on paper 41 Which of the following is NOT a possible sign of a chemical reaction? a. Formation of a gas b. Formation of a precipitate c. Color or temperature change d. Change in physical appearance 42 Which of the following is an example of a chemical change? a. Water evaporating b. Wood burning c. Ice cream melting d. Salt dissolving in water 43 Carbon combines with oxygen to form carbon dioxide. What is the word equation for this chemical reaction? a. Carbon dioxide carbon + oxygen b. Carbon carbon dioxide + oxygen c. Carbon + oxygen d. Carbon dioxide + oxygen carbon dioxide carbon 44 Which of the following may happen during a physical change? a. New substances are formed b. Reactants form products c. A precipitate is formed d. The temperature might increase 45 Identify the product(s) in this chemical reaction: Na + Cl NaCl a. Sodium (Na) b. Chlorine (Cl) c. Salt (NaCl) 46 What does the law of conservation of matter state? a. The total mass of the reactants is greater than the total mass of the products b. The total mass of the reactants is less than the total mass of the products c. The total mass of the reactants equals the total mass of the products d. Mass can be created and destroyed 47 The reactants involved in a chemical reaction are shown: Na2O + 2H2O How many O atoms must be present in the product that forms? a. 1 b. 2 c. 3 d. 4 48 Hydrogen gas can be produced by the following equation: Mg + 2HCl MgCl2 + H2 If hydrogen atoms cannot be created, what is the source of the atoms in the hydrogen gas? a. Mg b. HCl c. Both Mg and HCl d. Neither Mg nor HCl 49 How many grams of oxygen is required for this reaction to occur? 2CH4 + 3O2 2CO + 4H2O 16 g ? 44 g 36 g a. 16 b. 36 c. 44 d. 64 50 What number correctly balances the following reaction? N2 + ?O2 2NO2 a. 1 b. 2 c. 3 d. 4 51 What is the mass of rust that forms in this reaction? 100 g Iron + 43 g Oxygen ? g Rust a. 43 g b. 100 g c. 143 g d. 286 g 52 Which of the following is an endothermic change? a. Paper burning b. Bomb exploding c. Ice + energy d. O2 + glucose liquid water CO2 + H2O + energy 53