* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download presentation source
Cardiac contractility modulation wikipedia , lookup
Coronary artery disease wikipedia , lookup
Quantium Medical Cardiac Output wikipedia , lookup
Rheumatic fever wikipedia , lookup
Heart failure wikipedia , lookup
Arrhythmogenic right ventricular dysplasia wikipedia , lookup
Lutembacher's syndrome wikipedia , lookup
Myocardial infarction wikipedia , lookup
Atrial fibrillation wikipedia , lookup
Electrocardiography wikipedia , lookup
Congenital heart defect wikipedia , lookup
Dextro-Transposition of the great arteries wikipedia , lookup
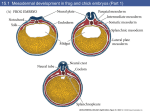
Developmental & Physiological Aspects of the Embryonic Chicken Heart Patrick Day John Schreier Biology 240W Spring 2000 Introduction Cellular migrations, fusions and specific differentiations are all integral parts of the development of the embryonic chicken heart. The embryonic chicken heart begins its development on the ventral surface from the fusion of paired pre-cardiac mesodermal tubes. These tubes are located on either side of the developing foregut. Introduction These paired heart vesicles start to fuse at the head (anterior end) and continue to fuse posteriorly to form a continuous tube. This happens between 25 to 30 hours of incubation. After fusion is complete, the heart tube can be identified from the anterior to posterior as conotruncus, ventricle, atrium, and sinus venosus. The heart begins to beat after the paired heart vesicles fuse. The sinus venosus in the fused heart tube becomes the embryonic pacemaker. Introduction Blood flows fom the sinus venosus to the conotruncus. The heart tube bends to form an S-shape. The ventricle bulges to the right. This happens after 33 hours of development. Introduction By 48 hours, the sinus venosus and atrium are moved to a position anterior and dorsal to the ventricle and conotruncus. Because the heart has folded upon itself, the ventricle is now Ushaped and the blood flows posteriorly and then makes a sharp turn to flow anteriorly. Introduction After 72 hours, the atrium prepares for division into the right and left atria by expanding. At this time, communication between the sinus venosus and the atrium is via the right side of the atrium. This is the first indication that the sinus venosus is becoming a part of the future right atrium. Introduction When the atrium and ventricle each divide into a pair of chambers, the sinus venosus, which is incorporated into the right atrium, gives rise to the sinoatrial (SA) node or the mature pacemaker. The Experiment We made the observation that the heart rate of a chick embryo is relatively constant when the embryo is kept in sterile saline solution and at a steady warm temperature of 37OC. After some background research, it was found that caffeine directly stimulates myocardial tissue, the “pacemaker” of the heart, and therefore has a direct effect on the rate and force of contraction of the heart. The Experiment We hypothesized that the heart rate of the chicken embryo will escalate with the addition of caffeine. We also hypothesized that the heart rate will increase proportionally with increasing concentrations of caffeine added. We hypothesized this because caffeine inhibits the activity of phosphodiesterase, which breaks down cyclic AMP. This increase in the amount of cyclic AMP affects the sympathetic nerves in the heart because it is a second messenger that causes the SA node to tell the heart to beat faster, which results in an increased force of ventricular contraction and accelerated heart rate. Methods 1. Three different 10 mL solutions of 3%, 0.3% and 0.03% caffeine respectively were prepared. 2. The 72-hour chick embryo/egg was then windowed as outlined in the lab handout. 3. The in vivo heart rate was then measured and recorded three times in rapid succession. 4. Next, the first solution of 0.03% diluted caffeine was added using a pipette to the in vivo chick embryo. Methods 5. The resulting heart rate was measured and recorded six times in rapid succession. 6. Steps 2-5 were then repeated using the rest of the diluted solutions (0.3% and 3%) and new in vivo chick embryos. 7. Next, a new 72-hr. chick embryo was then explanted using the methodology outlined in the lab handout. 8. The in vitro heart rate was then measured and recorded three times in rapid succession. Methods 9. Next, the first solution of 0.03% diluted caffeine was added using a pipette to the in vitro chick embryo. 10. The resulting heart rate was then measured and recorded six times in rapid succession. 11. Steps 7-10 were repeated using the rest of the diluted solutions (0.3% and 3%) and new in vitro chick embryos. Control Heart Rate (in vivo, in vitro) The control for the in vivo embryo was 120 bpm (depicted with blue horizontal line on graph). The control for the in vitro embryo was 103 bpm (depicted with red horizontal line on graph). Results (in vivo) Results (in vitro) Discussion For the in vivo embryo, our hypothesis was supported. The heart rate increased with the concentration of caffeine added because it inhibits the enzyme phosphodiesterase, which caused a build up of cyclic AMP. This build up excited the sympathetic nerves in the heart and increased the heart rate. Discussion For the in vitro embryo, our hypothesis was not supported. The results that were observed were too erratic to be considered supportive. Our interpretation of this inconsistent data is that the addition of the more concentrated caffeine resulted in a sudden increase in heart rate…so sudden it shocked the heart into fibrillation and eventually cardiac arrest. Future Experiments To get more desired results, new and improved experiments could be carried out. For example, the dilutions of caffeine administered could be less potent and segmented into a less dramatic increase of concentration. Another variation of the experiment above could be to carry it out on a older chick embryo (96hr). The advantage here is that the heart would be more developed and less prone to cardiac arrest. References McLaughlin, J.S. and E.R. McCain. Developmental and Physiological Aspects of the Chicken Embryonic Heart. 10 Februaury 1999. http://www.lv.psu.edu/jxm57/chicklab. Sadler, M.J., J.J. Strain, and B. Caballero. 1999. Encyclopedia of Human Nutrition, 1:A-D. San Diego: Academic Press.