* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Chapter 22 REDOX
Metallic bonding wikipedia , lookup
Chemical reaction wikipedia , lookup
Water splitting wikipedia , lookup
Marcus theory wikipedia , lookup
Artificial photosynthesis wikipedia , lookup
Atomic theory wikipedia , lookup
Oxidative phosphorylation wikipedia , lookup
Theory of solar cells wikipedia , lookup
Oxidation state wikipedia , lookup
Electrolysis of water wikipedia , lookup
History of electrochemistry wikipedia , lookup
Photoredox catalysis wikipedia , lookup
Metalloprotein wikipedia , lookup
Evolution of metal ions in biological systems wikipedia , lookup
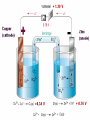
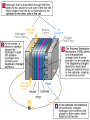
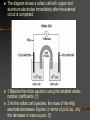
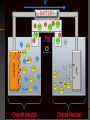
Chapter 22 REDOX Chapter 22 REDOX The Meaning of Oxidation and Reduction Oxidation Numbers Balancing Redox Equations Ch 22.1 The Meaning of Oxidation and Reduction Oxygen in Redox Reactions Electron Transfer in Redox Reactions Corrosion Oxygen in Redox Reactions Oxidation – the combination of an element with oxygen to produce oxides Oxygen in Redox Reactions Burning Oxygen in Redox Reactions Bleaching Oxygen in Redox Reactions Rusting Reduction Reduction – the loss of oxygen from a compound Redox Reactions Reduction and Oxidation always occur together 2Fe2O3(s) + 3C(s) 4Fe(s) + 3CO2(g) reduction oxidation Electron Transfer in Redox Reactions Oxidation – loss of electrons, gain oxygen Reduction – gain of electrons, loss of oxygen “LEO the lion goes GER” LEO – Lose electrons oxidation GER – Gain electrons reduction Electron Transfer in Redox Reactions Oxidation: Mg Mg2+ + 2e Loss of electrons Reduction: S + 2e- S2 Gain of electrons Corrosion Corrosion 2Fe(s) + O2(g) + 2H2O(l) 2Fe(OH)2(s) 4Fe(OH)2(s) + O2(g) + 2H2O(l) 4Fe(OH)3(s) Corrosion of iron Corrosion Some metals completely corrode Some metals form a protective coating Iron Aluminum Some metals do not corrode at all Gold Chapter 22.3 Balancing Redox Reactions Identifying Redox Reactions Using Oxidation Number Changes Using Half Reactions Identifying Redox Reactions Two types of reactions: REDOX – electrons are transferred Everything else: single replacement, double replacement, combustion, …. NO transfer of electrons Identifying Redox Reactions REDOX – the oxidation number of an element changes N2(g) + O2(g) 2NO(g) Using Oxidation Number Changes Fe2O3(s) + CO(g) Fe(s) + CO2(g) Step 1 – Assign oxidation numbers to all atoms in the equation Fe2O3(s) + CO(g) Fe(s) + CO2(g) +3 -2 +2 –2 0 +4 -2 Using Oxidation Number Changes Step 2 – Identify which atoms are oxidized and reduced Fe2O3(s) + CO(g) Fe(s) + CO2(g) +3 -2 +2 -2 0 +4 -2 Iron – reduced, Carbon - oxidized Using Oxidation Number Changes Step 3 – Use a bracket line to connect the atoms undergoing oxidation and one to connect the lines undergoing reduction +2 Fe2O3(s) + CO(g) Fe(s) + CO2(g) -3 Using Oxidation Number Changes Make the total increase in oxidation number equal to the total decrease in oxidation number by using appropriate coefficients 3 x (+2) = 6 Fe2O3(s) + CO(g) Fe(s) + CO2(g) 2 x (-3) = - 6 Using Oxidation Number Changes Step 5 – Finally make sure the equation is balanced for both atoms and charge Fe2O3(s) + 3CO(g) 2Fe(s) + 3CO2(g) FRH – Flameless Ration Heater Mg + H2O Mg(OH)2 + H2 + Heat Problem: Mg forms a coating from corrosion – MgO – which is not water soluble, prevents the above reaction from happening Solution: Add NaCl and Fe to the mix, breaks down the MgO and allows the reaction to happen Chapter 23 Electrochemistry Electrochemical Cells Half Cells and Cell Potentials Electrolytic Cells Electrochemical Cells The Nature of Electrochemical Cells Voltaic Cells Dry Cells Lead Storage Batteries Fuel Cells The Nature of Electrochemical Cells Zn(s) + Cu2+(aq) Zn2+(aq) + Cu(s) The Nature of Electrochemical Cells The zinc bar becomes copper plated Zinc loses electrons and dissolves slowly Copper gains electrons and becomes a solid Oxidation: Zn(s) Zn2+(aq) + 2eReduction: Cu2+(aq) + 2e- Cu(s) The Nature of Electrochemical Cells Reference Table J Look at any two metals, the metal that is higher on the table is the one that is more readily oxidized Electrochemical Cell Any device that converts chemical energy into electrical energy or electrical energy into chemical energy REDOX reactions must occur If an electrochemical cell is to be used for electrical energy, the two half reactions must physically be separated Voltaic Cell Alessandro Volta (1745 – 1827) First electrochemical cell Voltaic Cell Convert chemical energy into electrical energy Half Cell – part of a voltaic cell, consists of a metal rod in a solution of ions Salt Bridge – Separates half cells, tube containing a strong electrolyte (can also use a porous plate) Voltaic Cell Anode – the anode where oxidation occurs Cathode – the cathode where reduction occurs Dry Cell A voltaic cell in which the electrolyte is a paste Alkaline Battery Improved dry cell, the Zinc electrode doesn’t corrode as fast Lead Storage Batteries A group of cells connected together Fuel Cell Voltaic cell in which a fuel substance undergoes oxidation Do not have to be recharged Oxidation: 2H2(g) + 4OH-(aq) 4H2O(l) + 4eReduction: O2(g) + 2H2O(l) + 4e- 4OH-(aq) http://automobiles.honda.com/fcx-clarity/ http://www.pbs.org/wgbh/nova/sciencenow /3210/01.html Hydrogen Refueling Stations The diagram shows a voltaic cell with copper and aluminum electrodes immediately after the external circuit is completed. 1 Balance the redox equation using the smallest wholenumber coefficients. [1] 2 As this voltaic cell operates, the mass of the Al(s) electrode decreases. Explain, in terms of particles, why this decrease in mass occurs. [1] Answers 3 Cu2+ (aq) + 2 Al(s) 3 Cu(s) + 2 Al3+ (aq) Aluminum particles are losing electrons and becoming aluminum ions that are entering the solution. It allows migration of ions, maintains neutrality, prevents polarization Electrolytic Cells An electrochemical cell used to cause chemical change through the application of electrical energy (electrical energy is added) Differences Voltaic (Galvanic) Cells Electrolytic Cells Flow of electrons is spontaneous Flow of electrons is pushed by an outside power source Anode negative Cathode positive Anode positive Cathode negative Similarities Voltaic (Galvanic) Cells and Electrolytic Cells Electrons flow from anode to cathode Reduction – cathode Oxidation – anode Electroplating is an electrolytic process used to coat metal objects with a more expensive and less reactive metal. The diagram below shows an electroplating cell that includes a battery connected to a silver bar and a metal spoon. The bar and spoon are submerged in AgNO3(aq). Explain why AgNO3 is a better choice than AgCl for use in this electrolytic process. [1] Explain the purpose of the battery in this cell. [1] Acceptable responses include, but are not limited to: Silver nitrate produces more ions than silver chloride in water. AgNO3 readily dissolves in H2O; AgCl dissolves only slightly in H2O. Acceptable responses include, but are not limited to: The battery provides the electrical energy necessary for the reaction to occur. The apparatus shown in the diagram consists of two inert platinum electrodes immersed in water. A small amount of an electrolyte, H2SO4, must be added to the water for the reaction to take place. The electrodes are connected to a source that supplies electricity. What type of electrochemical cell is shown? [1] What particles are provided by the electrolyte that allow an electric current to flow? [1] Electrolytic or electrolysis. Acceptable responses include, but are not limited to: Ions, charged particles, H3O+, SO42– Because tap water is slightly acidic, water pipes made of iron corrode over time, as shown by the balanced ionic equation below: 2Fe + 6H+ 2Fe3+ + 3H2 Explain, in terms of chemical reactivity, why copper pipes are less likely to corrode than iron pipes. [1] Acceptable responses include, but are not limited to: Copper is less reactive than iron. Cu below H2 on Table J