* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Oral Presentation 4 - Research
Management of acute coronary syndrome wikipedia , lookup
History of invasive and interventional cardiology wikipedia , lookup
Antihypertensive drug wikipedia , lookup
Coronary artery disease wikipedia , lookup
Lutembacher's syndrome wikipedia , lookup
Quantium Medical Cardiac Output wikipedia , lookup
Atrial septal defect wikipedia , lookup
Dextro-Transposition of the great arteries wikipedia , lookup
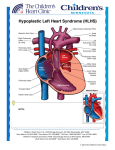
Pulmonary Flow Resistive Device Taya Furmanski Albert Attia Advisor: Thomas Doyle, M.D. April 9, 2003 Background Hypoplastic Left Heart Syndrome (HLHS) is a condition in which the patient is missing his/her left ventricle 1440 babies are born each year with HLHS Approximately 75% 3-year survival rate No medical treatment for HLHS Only options are operation (reconstruction) or transplantation 300 patients with HLHS are seen at VUMC per year The Problem Inadequate systemic blood flow Amount of O2 delivered to the organs decreases significantly “Blue Baby” Flow schematic How to Solve the Problem Place nozzle in pulmonary arteries (see figure for location) Device will act as resistor Decrease in pulmonary blood flow will cause increase in systemic blood flow Eliminates first two steps of reconstructive surgery Duration of implantation in heart = 6-8 months Schematic of Flow with and without Device Implanted <1 L/min 1-3 L/min Systemic Artery 2-3 L/min Systemic Artery 2-3 L/min Pulmonary Artery Pulmonary Artery 3-5 L/min Right Ventricle WITHOUT DEVICE 1 L/min 1 L/min Pulmonary Artery Pulmonary Artery 3-5 L/min Right Ventricle WITH DEVICE Dimensions of the Nozzle Calculations by Craig Russell (ME student) Theories required to solve problem Conservation of mass Conservation of momentum Dimension of end of nozzle still to be determined Pressure drop across nozzle required Will conduct pressure tests to solve for this unknown Pulmonary artery pressure ~20 mmHg Alternate Solutions Place nozzle inside stent Use bow-tie shaped stent (see figure) Placing a mesh-like device in the pulmonary arteries Problems With Alternate Solutions Extremely difficult to place in the artery Placement also a problem Would cause hemolysis (tiny holes would damage red blood cells) What We Need - Modeling Prototype can be tested through model to determine effectiveness In vitro model to simulate flow through blood vessels Computer model would allow variables to be altered easily to determine the optimal dimensions of the device What We Need - Materials & Assistance Use Vanderbilt shop to mold conical device Use NCIIA to produce working prototype Possibly have a company produce Nitinol prototype Use materials to create physical model that accurately portrays operation of device Assistance of mechanical engineering students (Craig Russell and Chris Owen) and professor (Dr. Mark Stremler) for fluid dynamics calculations Find experienced programmer to develop computer modeling system or use one currently in existence Why Nitinol? Biocompatible Memory wire—can be molded to desirable shape Can be elongated to fit into catheter, enabling insertion What We Have Accomplished Thus Far… In-depth research of HLHS Several meetings with Dr. Doyle to discuss the problem and possible solutions Finalizing a design plan Create a plan of attack: start simple and increase complexity Ordered and received Nitinol wire Calculations of fluid dynamics Finalized method of implantation Obtained materials necessary to test physical model What We Have Yet to Do… Produce prototype of device Test prototype Use Mechanical Engineering Energetics lab to test pressure drop across device Pressure drop calculations will allow proper calculation of dimension of the nozzle Create or find computer model simulation of cardiovascular system Conclusions Device will decrease blood flow to pulmonary arteries, Increase systemic bloodflow Bypass first two reconstructive (Norwood) surgeries Nitinol is an adequate material for this device Problem more complex than initially anticipated Fluid dynamics calculations Unmeasured pressure difference Device never created before Recommendations Continue pressure testing In vivo testing i.e. pigs, sheep Human clinical testing IRB approval FDA approval References 1. 2. 3. 4. 5. 6. Barber, Gerald. Hypoplastic Left Heart Syndrome. Structural Congenital Defects, section 3. www.ucch.org/sections/cardio/new/hlhs.html; date accessed: January 30, 2003. web1.tch.harvard.edu/chnews/03-15-02/fetalcath.html; date accessed: February 10, 2003. Dr. Thomas Doyle; Vanderbilt University Medical Center. http://www.nemours.org/no/ncc/cardiac/crd1524.html; date accessed: February 25, 2003. http://www.academicradiology.com/AR_2001/Jun01/5c 060100484p.PDF; date accessed: April 8, 2003.