* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download monitoring_growth
Survey
Document related concepts
Transcript
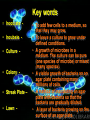
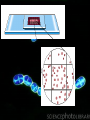
Monitoring growth Learning objective: To be able to describe ways of growing bacteria and ways of monitoring their growth viable plate count per mm3 medium Questions 250 200 150 glucose only lactose only lactose and glucose 100 50 0 0 50 100 150 200 250 sampling time following innoculation 300 Similarities • Lag phase. – Little increase in cell number; – Individual bacteria may be increasing in size; – Enzymes may be being synthesised to utilise the nutrient medium;max.2 • Log phase. – The population shows exponential phase; – Nutrient supply is not a limiting factor; • Stationary phase. – The population growth slows down; – The number of new cells formed is balanced by the number of cells dying; – Limiting factors, such a nutrient supply and metabolic wastes have started to influence further increase in population size; max 2 Differences • Lag phase for lactose alone longer; nutrient less readily available; • Growth of population less than with glucose; fewer cells and longer to increase;max.2 • Growth with lactose and glucose gives a greater population; • Has a second lag; around 100-120 minute and then increases again; • Uses glucose first as growth curve similar to glucose alone; and then uses lactose;max 3 • Death phase is occurring for glucose alone as all the energy source/glucose has run out.1 Growing Microbes • Microbes for experiments can be obtained from natural sources (e.g. soil, food, skin, water, air), or bought from suppliers in agar slopes. • You don't need much, since a dot may contain millions of viable cells, each of which could grow into a whole colony. Medium • The mixture that the microbes are grown (cultured) on. • The medium must contain all the nutrients (sugars, minerals, proteins) needed for the microbes to grow. • By adjusting these nutrients, the medium can be made selective for one type of microbe. • Culture media can be made up by mixing together known amounts of specific chemicals (a defined or synthetic medium), or they can be made from a natural source such as boiled meat or yeast extract, which generally contains the nutrients required by most microbes (an undefined or complex medium). Nutrient medium • A cheap general-purpose complex medium used for most school experiments. Broth • A liquid medium (i.e. without agar). Agar • Agar is mixed with a liquid medium to make a solid medium, which is very useful to observe, separate and store bacteria cultures. • A solid medium in a petri dish is known as an agar plate, while a solid medium in a Universal bottle is called an agar slope. • Agar is actually a polysaccharide extracted from seaweed. • It melts at 41°C (so can be incubated at 37°C without melting), is reasonably transparent, and is not broken down by microbes, so it remains solid. Aseptic transfer • Also called aseptic technique. • The transfer of a sample of bacteria from one vessel to another. • This is the most common and basic technique and is used in almost all micro-biological experiments. • The bacteria are usually transferred using a wire or glass inoculating loop, which can carry a tiny volume of culture (10 µl) or a scraping of cells from an agar plate. • Larger volumes are transferred using a sterile syringe or pipette. Key words • Inoculate • Incubate • Culture • Colony • Streak Plate – • Lawn - • To add few cells to a medium, so that they may grow. • To leave a culture to grow under defined conditions. • A growth of microbes in a medium. The culture can be pure (one species of microbe) or mixed (many species). • A visible growth of bacteria on an agar plate containing many millions of cells. • A method of inoculating an agar plate with bacteria so that the bacteria are gradually diluted. • A layer of bacteria growing on the surface of an agar plate. Measuring the Growth of Microbes • Growth of cells in a liquid culture is generally measured by simply counting the number of cells. • There are various techniques for doing this. Some give total cell counts, which include both living and dead cells, while others give viable cell counts, which only include living cells. Haemocytometer • This counts the total cells by observing the individual cells under the microscope. • This is reasonably easy for large cells like yeast, but is more difficult for bacterial cells, since they are so small. • The cell counter (or haemocytometer) is a large microscope slide with a very accurate grid drawn in the centre. • The grid marks out squares with 1 mm, 0.2 mm and 0.05 mm sides. • There is an accurate gap of 0.1 mm between the grid and the thick coverslip, so the volume of liquid above the grid is known. • The number of cells in a known small volume can thus be counted, and so scaled up. The units are cells per cm3 . For example: • A 0.2 mm square has an average of 80 cells in a 1000x dilution • Volume above square = 0.2 x 0.2 x 0.1 = 0.004 mm³ • 80 cells in 0.004 mm³ = 20 000 cells per mm³ in the diluted suspension • which is 20 000 x 1000 = 2 x 107 cells per mm³ of undiluted suspension • or 2 x 1010 cells per cm³ Turbidometry • This technique also counts the total cells. • It is quicker than using a haemocytometer, but less accurate. • A sample of the liquid culture is placed in a cuvette in a colorimeter, and the absorbance of light is measured. • The greater the concentration of the cells, the more cloudy or turbid the liquid is, so the more light it scatters, so the less light is transmitted to the detector. • A wavelength of 600nm is normally used. • Although the absorbance scale of the colorimeter is used, light is not actually absorbed by the cells (as it is by pigment molecules), but scattered. • If the same sample is counted in a haemocytometer and its absorbance measured, than a calibration curve can be plotted. • From this calibration curve the concentration of cells can be read off for any absorbance. Dilution Plating • This technique counts viable cells. • A sequence of ten-fold dilutions is taken from the original culture flask, using sterile medium. • This is called a serial dilution, and allows large dilutions to be made using small volumes. • From each dilution a 1 cm³ sample is taken and spread evenly onto an agar plate. • Each viable cell in the sample will multiply and grow into a colony. • In most of the samples there will be too many colonies to count, but in one of the dilutions there will be a good number (20-200) of individual colonies. • From this we can calculate the concentration of viable cells in the original culture. For example: • Suppose there were 83 colonies in the x10 000 dilution agar plate. • How many viable cells would there have been per cm³ in the original culture? • There were 83 viable cells in the 1 cm³ sample of the x10 000 dilution • So there were 83 x 10 000 = 8.3 x 105 cells per cm³ in the original culture Questions