* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Chapter 3
Restoration ecology wikipedia , lookup
Habitat conservation wikipedia , lookup
Biodiversity action plan wikipedia , lookup
Biological Dynamics of Forest Fragments Project wikipedia , lookup
Photosynthesis wikipedia , lookup
River ecosystem wikipedia , lookup
Sulfur cycle wikipedia , lookup
Biosphere 2 wikipedia , lookup
Reconciliation ecology wikipedia , lookup
Sustainable agriculture wikipedia , lookup
Nitrogen cycle wikipedia , lookup
Theoretical ecology wikipedia , lookup
Renewable resource wikipedia , lookup
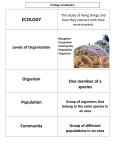
Chapter 3 Ecosystems: What Are They and How Do They Work? Core Case Study: Have You Thanked the Insects Today? Many plant species depend on insects for pollination. Insect can control other pest insects by eating them Figure 3-1 Core Case Study: Have You Thanked the Insects Today? …if all insects disappeared, humanity probably could not last more than a few months [E.O. Wilson, Biodiversity expert]. Insect’s role in nature is part of the larger biological community in which they live. THE NATURE OF ECOLOGY Ecology is a study of connections in nature. How organisms interact with one another and with their nonliving environment. Figure 3-2 Ecology ‣ Ecology: the study of the relationships between organisms & the abiotic (nonliving) and biotic (living) environment. The physical conditions influence the habitat in which an organism lives. These include: substrate CO 2 O 2 humidity sunlight temperature salinity pH (acidity) exposure altitude depth Nutrients Each abiotic (or physical) factor may be well suited to the organism or it may present it with problems to overcome. Universe Galaxies Solar systems Biosphere Planets Earth Biosphere Ecosystems Ecosystems Communities Populations Realm of ecology Organisms Organ systems Communities Organs Tissues Cells Populations Protoplasm Molecules Atoms Organisms Subatomic Particles Fig. 3-2, p. 51 Biological Complexity ‣ Living organisms can be studied at different levels of complexity. ‣ From least to most complex, these levels are (in an ecological context): Individual Population Community Ecosystem Biome Biosphere Biosphere Biome Ecosystem Community Population Individual Organisms and Species Organisms, the different forms of life on earth, can be classified into different species based on certain characteristics. Figure 3-3 Other animals 281,000 Known species 1,412,000 Insects 751,000 Fungi 69,000 Prokaryotes 4,800 Plants 248,400 Protists 57,700 Fig. 3-3, p. 52 Case Study: Which Species Run the World? Multitudes of tiny microbes such as bacteria, protozoa, fungi, and yeast help keep us alive. Harmful microbes are the minority. Soil bacteria convert nitrogen gas to a usable form for plants. They help produce foods (bread, cheese, yogurt, beer, wine). 90% of all living mass. Helps purify water, provide oxygen, breakdown waste. Lives beneficially in your body (intestines, nose). Populations, Communities, and Ecosystems Members of a species interact in groups called populations. Populations of different species living and interacting in an area form a community. A community interacting with its physical environment of matter and energy is an ecosystem. Populations A population is a group of interacting individuals of the same species occupying a specific area. The space an individual or population normally occupies is its habitat. Figure 3-4 Populations Genetic diversity In most natural populations individuals vary slightly in their genetic makeup. Figure 3-5 Community The population of all species living & interacting in an area. Ecosystems Ecosystems consist of nonliving (abiotic) and living (biotic) components. Figure 3-10 The Biosphere The biosphere is the region within which all living things are found on Earth, extending from the bottom of the oceans to the upper atmosphere. The biosphere is but one of the four separate components of the geochemical model along with the lithosphere, hydrosphere, and atmosphere. The Gaia Hypothesis maintains that the Earth is a single self-regulating complex evolving system. An example being the exchange of elements between oceans and land. THE EARTH’S LIFE SUPPORT SYSTEMS The biosphere consists of several physical layers that contain: Air Water Soil Minerals Life Figure 3-6 Biosphere Atmosphere Membrane of air around the planet. Stratosphere Lower portion contains ozone to filter out most of the sun’s harmful UV radiation. Hydrosphere All the earth’s water: liquid, ice, water vapor Lithosphere The earth’s crust and upper mantle. Oceanic Crust Atmosphere Vegetation Biosphere and animals Soil Crust Rock Continental Crust Lithosphere Upper mantle Asthenosphere Lower mantle Core Mantle Crust (soil and rock) Biosphere Hydrosphere (living and dead (water) organisms) Lithosphere Atmosphere (crust, top of upper mantle) (air) Fig. 3-6, p. 54 Habitat An organism’s habitat is the physical place or environment in which it lives. Organisms show a preference for a particular habitat type, but some are more specific in their requirements than others. Lichens, fungi & algae or bacteria, are found on rocks, trees, and bare ground. Most frogs, like this leopard frog, live in or near fresh water, but a few can survive in arid habitats. Habitat Needs Cover – shelter; trees, shrubs, etc. Water Nutrients Macronutrients Chemicals organisms need in large numbers to live, grow, and reproduce. Ex. carbon, oxygen, hydrogen, nitrogen, calcium, and iron. Micronutrients These are needed in small or even trace amounts. Ex. sodium, zinc copper, chlorine, and iodine. Ecological Niche The ecological niche describes the functional position of an organism in its environment. A niche comprises: the habitat in which the organism lives. the organism’s activity pattern: the periods of time during which it is active. the resources it obtains from the habitat. Adaptations Habitat Activity patterns Presence of other organisms Physical conditions The Fundamental Niche The fundamental niche of an organism is described by the full range of environmental conditions (biological and physical) under which the organism can exist. The realized niche of the organism is the niche that is actually occupied. It is narrower than the fundamental niche. This contraction of the realized niche is a result of pressure from, and interactions with, other organisms. Factors That Limit Population Growth Availability of matter and energy resources can limit the number of organisms in a population. Figure 3-11 Law of Tolerance The law of tolerance states that “For each abiotic factor, an organism has a range of tolerances within which it can survive.” Tolerance range Number of organisms Optimum range Unavailable Marginal niche niche Examples of abiotic factors that influence size of the realized niche Too acidic Too cold Preferred niche Marginal Unavailable niche niche pH Temperature Too alkaline Too hot Factors That Limit Population Growth The physical conditions of the environment can limit the distribution of a species. Figure 3-12 Population Growth Cycle Limited Resources A population can grow until competition for limited resources increases & the carrying capacity (C.C.) is reached. Typical Phases 1. The population overshoots the C.C. 2. This is because of a reproductive time lag (the period required for the birth rate to fall & the death rate to rise). 3. The population has a dieback or crashes. 4. The carrying capacity is reached. What Happens to Solar Energy Reaching the Earth? Solar energy flowing through the biosphere warms the atmosphere, evaporates and recycles water, generates winds and supports plant growth. Figure 3-8 Producers: Basic Source of All Food Most producers capture sunlight to produce carbohydrates by photosynthesis: Producers: Basic Source of All Food Chemosynthesis: Some organisms such as deep ocean bacteria draw energy from hydrothermal vents and produce carbohydrates from hydrogen sulfide (H2S) gas . Photosynthesis: A Closer Look Chlorophyll molecules in the chloroplasts of plant cells absorb solar energy. This initiates a complex series of chemical reactions in which carbon dioxide and water are converted to sugars and oxygen. Figure 3-A Sun Chlorophyll H2O Light-dependent Reaction Chloroplast in leaf cell O2 Energy storage and release (ATP/ADP) CO2 6CO2 + 6 H2O Lightindependent reaction Sunlight Glucose C6H12O6 + 6 O2 Fig. 3-A, p. 59 Consumers: Eating and Recycling to Survive Consumers (heterotrophs) get their food by eating or breaking down all or parts of other organisms or their remains. Herbivores • Primary consumers that eat producers Carnivores • Primary consumers eat primary consumers • Third and higher level consumers: carnivores that eat carnivores. Omnivores • Feed on both plant and animals. Decomposers and Detrivores Decomposers: Recycle nutrients in ecosystems. Detrivores: Insects or other scavengers that feed on wastes or dead bodies. Figure 3-13 Scavengers Longhorned beetle holes Decomposers Termite and Bark beetle Carpenter carpenter ant engraving galleries ant work Dry rot fungus Time progression Wood reduced to Mushroom powder Powder broken down by decomposers into plant nutrients in soil Fig. 3-13, p. 61 Aerobic and Anaerobic Respiration: Getting Energy for Survival Organisms break down carbohydrates and other organic compounds in their cells to obtain the energy they need. This is usually done through aerobic respiration. The opposite of photosynthesis Aerobic and Anaerobic Respiration: Getting Energy for Survival Anaerobic respiration or fermentation: Some decomposers get energy by breaking down glucose (or other organic compounds) in the absence of oxygen. The end products vary based on the chemical reaction: • • • • Methane gas Ethyl alcohol Acetic acid Hydrogen sulfide Two Secrets of Survival: Energy Flow and Matter Recycle An ecosystem survives by a combination of energy flow and matter recycling. Figure 3-14 Decomposition As plant or animal matter dies it will break down and return the chemicals back to the soil. This happens very quickly in tropical rainforest which results in low-nutrient soils. Grasslands have the deepest and most nutrient rich of all soils BIODIVERSITY Figure 3-15 Biodiversity Loss and Species Extinction: Remember HIPPO H for habitat destruction and degradation I for invasive species P for pollution P for human population growth O for overexploitation Biodiversity Loss and Species Extinction: Remember HIPPCO H for habitat destruction and degradation I for invasive species P for pollution P for human population growth C for Climate Change O for overexploitation Why Should We Care About Biodiversity? Biodiversity provides us with: Natural Resources (food water, wood, energy, and medicines) Natural Services (air and water purification, soil fertility, waste disposal, pest control) Aesthetic pleasure Solutions Goals, strategies and tactics for protecting biodiversity. Figure 3-16 ENERGY FLOW IN ECOSYSTEMS Food chains and webs show how eaters, the eaten, and the decomposed are connected to one another in an ecosystem. Figure 3-17 Food Webs Trophic levels are interconnected within a more complicated food web. Figure 3-18 Energy Flow in an Ecosystem: Losing Energy in Food Chains and Webs accordance with the 2nd law of thermodynamics, there is a decrease in the amount of energy available to each succeeding organism in a food chain or web. In Energy Flow in an Ecosystem: Losing Energy in Food Chains and Webs Ecological efficiency: percentage of useable energy transferred as biomass from one trophic level to the next. Figure 3-19 10% Rule We assume that 90% of the energy at each energy level is lost because the organism uses the energy. (heat) It is more efficient to eat lower on the energy pyramid. You get more out of it! This is why top predators are few in number & vulnerable to extinction. Productivity of Producers: The Rate Is Crucial Gross primary production (GPP) Rate at which an ecosystem’s producers convert solar energy into chemical energy as biomass. Figure 3-20 Net Primary Production (NPP) NPP = GPP – R Rate at which producers use photosynthesis to store energy minus the rate at which they use some of this energy through respiration (R). Figure 3-21 What are nature’s three most productive and three least productive systems? Figure 3-22 What Sustains Life on Earth? Solar energy, the cycling of matter, and gravity sustain the earth’s life. Figure 3-7 MATTER CYCLING IN ECOSYSTEMS Nutrient Cycles: Global Recycling Global Cycles recycle nutrients through the earth’s air, land, water, and living organisms. Nutrients are the elements and compounds that organisms need to live, grow, and reproduce. Biogeochemical cycles move these substances through air, water, soil, rock and living organisms. The Water Cycle Figure 3-26 Water’ Unique Properties There are strong forces of attraction between molecules of water. Water exists as a liquid over a wide temperature range. Liquid water changes temperature slowly. It takes a large amount of energy for water to evaporate. Liquid water can dissolve a variety of compounds. Water expands when it freezes. Effects of Human Activities on Water Cycle We alter the water cycle by: Withdrawing large amounts of freshwater. Clearing vegetation and eroding soils. Polluting surface and underground water. Contributing to climate change. The Carbon Cycle: Part of Nature’s Thermostat Figure 3-27 The Carbon Cycle Effects of Human Activities on Carbon Cycle We alter the carbon cycle by adding excess CO2 to the atmosphere through: Burning fossil fuels. Clearing vegetation faster than it is replaced. Figure 3-28 Carbon Cycling ‣ ‣ Carbon cycles as gaseous carbon is fixed in the process of photosynthesis and returned to the atmosphere in respiration. Burning fossil fuels Carbon may remain locked up in sinks or reservoirs that are biotic or abiotic for long periods of time, e.g. in the wood of trees, oceans or in fossil fuels such as coal or oil. ‣ ‣ Indirectly carbon forms carbonate deposits as carbon is removed from the atmosphere. (The carbonate is stored mostly in the marine ecosystem.) Humans have disturbed the balance of the carbon cycle through activities such as combustion and deforestation. Petroleum & Coal The Nitrogen Cycle: Bacteria in Action Nitrogen Cycle Most of the work done by bacteria & decomposers Process Product Formulas Nitrogen Fixation Ammonia N2 → NH4 Nitrification Nitrite then nitrate NH4 → NO2 →NO3 Assimilation Proteins: amino acids, DNA NO3 (some NH4) → uptake by plant Ammonification (Decay & waste products) Ammonia Plant & animal decay & excretions → NH4 Denitrification Nitrogen gas NO3 → N2 Nitrogen in the Environment ‣ Nitrogen cycles between the biotic and abiotic environments. The largest reservoir for nitrogen is the atmosphere and thus it is difficult to fix, bacteria play an important role in this transfer. Nitrogen-fixing bacteria are able to fix atmospheric nitrogen. Lightning can fix atmospheric nitrogen Nitrifying bacteria convert ammonia to nitrite, and nitrite to nitrate. Denitrifying bacteria return fixed nitrogen to the atmosphere. Atmospheric fixation also occurs as a result of lightning discharges. ‣ Humans intervene in the nitrogen cycle by producing and applying nitrogen (nitrates) fertilizers which is the main cause of eutrophication. Bacteria on the roots of legumes can fix nitrogen Effects of Human Activities on the Nitrogen Cycle We alter the nitrogen cycle by: Adding gases that contribute to acid rain. Adding nitrous oxide to the atmosphere through farming practices which can warm the atmosphere and deplete ozone. Contaminating ground water from nitrate ions in inorganic fertilizers. Releasing nitrogen into the troposphere through deforestation. Effects of Human Activities on the Nitrogen Cycle Human activities such as production of fertilizers now fix more nitrogen than all natural sources combined. Figure 3-30 The Phosphorous Cycle mining excretion Fertilizer Guano agriculture uptake by uptake by weathering autotrophs autotrophs leaching, runoff Dissolved Land Marine Dissolved in Soil Water, Food Food in Ocean Lakes, Rivers Webs Webs Water death, death, decomposition decomposition weathering sedimentation settling out uplifting over geologic time Rocks Marine Sediments Fig. 3-31, p. 77 Phosphorus Cycling Phosphorus cycling is very slow and tends to be local and stable; in aquatic and terrestrial ecosystems, phosphorous is a sedimentary cycle. Phosphorous is lost from ecosystems through run-off, precipitation, and sedimentation. A very small amount of phosphorus returns to the land as guano (manure of fish-eating birds). Weathering and phosphatizing bacteria return phosphorus to the soil. Deposition as guano… Loss via sedimentation… Often the Limiting factor in the ecosystem The phosphorous cycle has no real significant gas phase and under many conditions will form stable insoluble compounds Fertilizer production Effects of Human Activities on the Phosphorous Cycle We remove large amounts of phosphate from the earth to make fertilizer. We reduce phosphorous in tropical soils by clearing forests. We add excess phosphates to aquatic systems from runoff of animal wastes and fertilizers contributing to eutrophication The Sulfur Cycle Figure 3-32 Sulfur trioxide Water Acidic fog and precipitation Sulfuric acid Ammonia Oxygen Sulfur dioxide Ammonium sulfate Hydrogen sulfide Plants Dimethyl sulfide Volcano Industries Animals Ocean Sulfate salts Metallic sulfide deposits Decaying matter Sulfur Hydrogen sulfide Fig. 3-32, p. 78 Sulfur Cycling Sulfur is naturally occurring in rock or mineral forms and is a sedimentary cycle. Sulfur is an essential component of proteins and is important in determining the acidity of precipitation, surface water, and soil. Sulfur circulates through the biosphere as: hydrogen sulfide (H2S), sulfur dioxide (SO2), sulfate (SO42-), and elemental sulfur (S) Sulfur in petrol Molecular bridges in proteins Elemental sulfur Effects of Human Activities on the Sulfur Cycle We add sulfur dioxide to the atmosphere by: Burning coal and oil Refining sulfur containing petroleum. Convert sulfur-containing metallic ores into free metals such as copper, lead, and zinc releasing sulfur dioxide into the environment. HOW DO ECOLOGISTS LEARN ABOUT ECOSYSTEMS? Ecologist go into ecosystems to observe, but also use remote sensors on aircraft and satellites to collect data and analyze geographic data in large databases. Geographic Information Systems Remote Sensing Ecologists also use controlled indoor and outdoor chambers to study ecosystems Geographic Information Systems (GIS) A GIS organizes, stores, and analyzes complex data collected over broad geographic areas. Allows the simultaneous overlay of many layers of data. Figure 3-33 Critical nesting site locations USDA Forest Service USDA Private Forest Service owner 1 Private owner 2 Topography Forest Habitat type Wetland Lake Grassland Real world Fig. 3-33, p. 79 Systems Analysis Ecologists develop mathematical and other models to simulate the behavior of ecosystems. Figure 3-34 Systems Measurement Define objectives Identify and inventory variables Obtain baseline data on variables Data Analysis Make statistical analysis of relationships among variables Determine significant interactions System Modeling Objectives Construct mathematical model describing interactions among variables System Simulation System Optimization Run the model on a computer, with values entered for different Variables Evaluate best ways to achieve objectives Fig. 3-34, p. 80 Importance of Baseline Ecological Data We need baseline data on the world’s ecosystems so we can see how they are changing and develop effective strategies for preventing or slowing their degradation. Scientists have less than half of the basic ecological data needed to evaluate the status of ecosystems in the United Sates (Heinz Foundation 2002; Millennium Assessment 2005).