* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download 7-Tumor Suppressor genes, Oncogenes and Development The
Gene expression profiling wikipedia , lookup
Transcriptional regulation wikipedia , lookup
Promoter (genetics) wikipedia , lookup
Cell-penetrating peptide wikipedia , lookup
X-inactivation wikipedia , lookup
Molecular evolution wikipedia , lookup
Gene regulatory network wikipedia , lookup
Silencer (genetics) wikipedia , lookup
List of types of proteins wikipedia , lookup
Artificial gene synthesis wikipedia , lookup
Endogenous retrovirus wikipedia , lookup
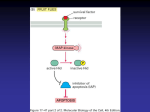
8 TH W E E K Gihan E-H Gawish, MSc, PhD Ass. Professor Molecular Genetics and Clinical Biochemistry KSU Development of Cancer 1. Defective chick points mechanisms allows errors in the cell duplication process to persist into the next generation and can lead to and regulated proliferation and the development of cancer 2. Two different types of mutations contribute to cancer formation: inactivating mutations in tumor suppressor genes and activating mutations in protooncogene. Tumor Suppressor Genes Inhibit Proliferation Promote Differentiation Stimulate Apoptosis Oncogenes Stimulate Proliferation Inhibit Differentiation Inhibit Apoptosis Tumor Suppressor Genes • Tumor Suppressor genes: are genes that act to inhibit cell proliferation and tumor development. If Tumor Suppressor Gene is Mutated Inactivated It will lead to cell transformation Normal transformed Oncogenes = gain of function Tumor suppressor = loss of function • First discovered in 1960s by Henry Harris. • Harris fused tumor cells with normal cells and discovered some of the hybrid cells were normal. • Harris hypothesized that the normal cells contained gene products that suppressed uncontrolled cell proliferation. Sporadic retinoblastoma – 60% of retinoblastoma cases. – Develops in children with no family history. – Occurs in one eye. Hereditary retinoblastoma – 40% of retinoblastoma cases. Retinoblastoma (Rb) caused by mutated Rb gene – Onset typically is earlier than sporadic cases. – Multiple tumors involving both eyes. Two mutations are required for the development of retinoblastoma. • Sporadic retinoblastoma – Child starts with two wild type alleles (RB+/RB+). – Both alleles must mutate to produce the disease (RB/RB). – Probability of both mutations occurring in the same cell is low; only one tumor forms (e.g., one eye). • Hereditary retinoblastoma – Child starts with heterozygous alleles (RB/RB+). – Only one mutation is required to produce disease (RB/RB). – Mutations resulting in loss of heterozygosity (LOH) are more probable in rapidly dividing cells, and multiple tumors occur (e.g., both eyes). •Tumor suppressors must be inactivated • This means both copies must be lost/mutated Hereditary cancer is caused by the inheritance of one copy of a defective tumor suppressor tumor-suppressor gene Normal growthinhibiting protein Cell division under control Mutated tumor-suppressor gene Defective, nonfunctioning protein Cell division not under control Functions of Tumor Suppressor genes 1. Antagonize the action of oncogenes – eg. p53 is activated by oncogenes. p53 protects against cancer by inducing cell cycle arrest and/or apoptosis myc p53 cell growth Functions of Tumor Suppressor genes 2. Block proliferation: – Cell cycle inhibitors: eg. Rb – blocks entry into S phase by binding to and inhibiting RB. INK-4 gene: that produces P16 that inhibits cdk4/cyclin D action ( to phosphorylate Rb gene to inactivate it’s action) – Repressor transcription factors: e.g.; WT1 is a repressor that appears to suppress transcription factor ( Insulin like growth factor) which will contribute in the development of tumor. – Activator transcription factors: e.g.; SMAD family that are activated by TGF-β, leading to inhibition of cell proliferation . P53: that produces P21 that has the same action of P16 in inhibiting the action of cdk/cyclin. Functions of Tumor Suppressor genes 3. Induce apoptosis: – Form of cell suicide. A cell which has lost growth control will often undergo apoptosis. – Cell damage or ‘stress’ can also lead to apoptosis. – p53 is a critical regulator of apoptosis. Transcription factor which activates pro-apoptotic molecules p53 Mutations • Most commonly mutated gene in cancers (50% of total). • When p53 is mutated, DNA-damaged cells are not arrested in G1 and DNA repair does not take place. • This failure to arrest DNA-damaged cells will be repeated in subsequent cell cycles permitting other mutations to accumulate, culminating in neoplastic transformation... tumor formation and cancer. p53 – the guardian of the genome Regulation of the cell cycle Functions of Tumor Suppressor genes 4. DNA Repair – DNA repair prevents the accumulation of mutations – Defects in DNA repair genes leads to genomic instability – Accelerates the activation of oncogenes and the loss of tumor suppressors – Many cancer prone syndromes associated with defects in DNA repair, BRCA1, ATM, MRE11, NBS, Breast Cancer Tumor Suppressors • A small proportion of breast cancer is heritable. Two genes are associated with predisposition to breast cancer. – BRCA1 on chromosome 17 – BRCA2 on chromosome 13 • Normal function of both is in repair of ds DNA breaks. • Tumor suppressor genes – inhibit oncogenes – suppress proliferation – Induce cell death – repair DNA – prevent mutation • These are “loss of function” or recessive mutations. • Responsible for hereditary forms of cancer • Being heterozygous enhances the probability of cancer but this will require a mutation in the corresponding other allele. e.g., it need to be homozygous for the gene. Oncogenes • Gene that can cause cancer. It is a sequence of DNA that has been altered or mutated from its original form, the protooncogene. • Proto-oncogenes promote the specialization and division of normal cells. A change in their genetic sequence can result in uncontrolled cell growth. • Dominant mutation: one copy is sufficient to cause cancer. In humans, proto-oncogenes can be transformed into oncogenes in three ways: • point mutation (alteration of a single nucleotide base pair) • translocation (in which a segment of the chromosome breaks off and attaches to another chromosome), • or amplification (increase in the number of copies of the proto-oncogene).. Inherited mutations of oncogenes • A few cancer syndromes are caused by inherited mutations of proto-oncogenes • Multiple endocrine neoplasia type 2 is caused by an inherited mutation in the gene called RET medullary cancer of the thyroid. • Inherited mutations in the gene called KIT cause hereditary gastrointestinal stromal tumors (GIST). • Inherited mutations in the gene called MET cause hereditary papillary renal cancer. Acquired mutations of oncogenes • Most cancer causing mutations involving oncogenes are acquired, not inherited. • They generally activate oncogenes by chromosome rearrangements, gene duplication, or mutation. For example, a chromosome rearrangement leads to formation of the gene called BCR-ABL. This leads to the disease chronic myeloid leukemia (CML). Chromosomal Translocation that creates Philadelphia Chromosome BCR-ABL Oncogene: Breaks in ABL Gene of Chromosome 9 and BCR Gene of Chromosome 22 Fusion Protein causes Chronic Myelogenous Leukemia History of oncogene • Oncogenes were first discovered in certain retroviruses and were later identified as cancer-causing agents in many animals • First link between viruses and cancer proposed by Francis Peyton Rous in 1910 (Nobel Prize, 1966): cell-free extracts from chicken tumors injected into healthy chickens caused new tumors. Rous Sarcoma Virus (RSV) • Discovered by Harold Varmus and Bishop, 1975-76 (Nobel Prize, 1989). • A transforming retrovirus: a cancer-causing single-stranded RNA virus that uses reverse transcriptase enzyme to make ssDNA, then ds DNA, which integrates into host DNA. • Note: not all oncogenes caused by viruses. • 100’s of oncogenes now known. • Human T-cell leukemia virus (HTLV) is a human RV; codes a TF. Southern Blots Probed with viral src Gene Revealed Cellular Origin of Oncogenes Infected chicken Uninfected chicken (Negative Control) v-src c-src Proto-oncogene SURPRISE! Origin of Transforming Retroviruses Capsid protein Reverse Transcriptase Envelope Protein Mutation creates oncogene Ras Proto-oncogene • Mutated in 30% of all cancers. • A “molecular switch” in the signal transduction pathway leading from growth factors to gene expression controlling cell proliferation: GF receptor Ras TF target genes growth. • A single amino acid change in Ras protein can cause constant stimulation of the pathway, even in the absence of growth factors. Cancers Usually Result from a Series of Mutations in a Single Cell • Colon Cancer: oncogene oncogene Tumor suppressors Tumor Progression: Evolution at the Cellular Level Benign tumor (polyp in epithelial cells) is confined by basal lamina; then additional mutation occurs. Malignant tumor (carcinoma in epithelial cells) grows very fast, becomes invasive, and metastasizes. Some of the more important oncogenes include: • ras (a signal transduction molecule), • myc (a transcription factor), • src (a protein tyrosine kinase), • HER-2/neu, also called erbB-2 (a growth factor receptor), • Bcl-2 (a membrane associated protein that prevents apoptosis).