* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Migration Medicine
Vectors in gene therapy wikipedia , lookup
Epigenetics of human development wikipedia , lookup
Epigenetics in learning and memory wikipedia , lookup
Protein moonlighting wikipedia , lookup
Epigenetics of neurodegenerative diseases wikipedia , lookup
Polycomb Group Proteins and Cancer wikipedia , lookup
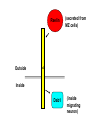
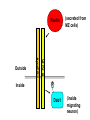
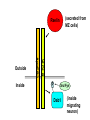
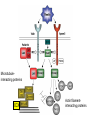
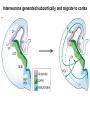
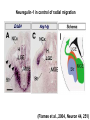
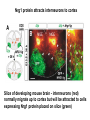
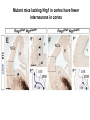
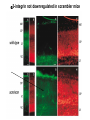
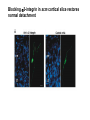
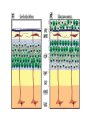
Cell migration in the cerebral cortex How does it work? What happens when it goes wrong? Story of interplay between basic and clinical science Inside-out programme of neurogenesis Somal translocation Glial-guided locomotion “Reeler” mutant mice - Mice are ataxic, fall over a lot - Lack cerebellum - Layers in cerebral cortex are inverted Inverted cortical layers in reeler mutants “Reeler” mutation mapped to gene, named Reelin. - Encodes large secreted protein - Specifically expressed by Cajal-Retzius cells in marginal zone of developing cortex - Mutations in the human Reelin gene result in Lissencephaly with Cerebellar Hypoplasia Lissencephaly with Cerebellar Hypoplasia (OMIM: 257320) - Autosomal recessive - Simplified folding of cortex (“smooth” cortex) - Cerebellum very underdeveloped - Severe ataxia, mental retardation and epilepsy “Scrambler” mutant mice show same behavioural and anatomical defects as Reeler mice - Mapped to gene Dab1 - Encodes cytoplasmic “adaptor” protein - Link cytoplasmic tails of transmembrane proteins to intracellular signaling proteins => Maybe in same biochemical pathway as Reelin? What’s in between them? Reelin ? Outside (secreted from MZ cells) Inside Dab1 (inside migrating neuron) Dab1 protein found to bind to cytoplasmic tails of two transmembrane proteins (related to each other): - VLDLR and ApoER2 (very low density lipoprotein receptor and ApoE receptor 2) - These proteins found to bind Reelin through their extracellular domains - Binding of Reelin leads to phosphorylation of Dab1 protein Reelin VLDLR Inside ApoER2 Outside (secreted from MZ cells) P Dab1 (inside migrating neuron) What happens when these genes are mutated? - Knock out VLDLR in mice: very little effect - Knock out ApoER2 in mice: very little effect - Make double KO: looks just like reeler mutants => These proteins are redundant in this process Upon Reelin binding to VLDLR or ApoER2: - Dab1 recruited - Dab1 phosphorylated by Src or Fyn (Src;Fyn double mutant has same phenotype as Dab1) - Signaling to cytoskeleton - Phosphorylated Dab1 degraded (entire complex including receptors endocytosed and degraded) (secreted from MZ cells) Reelin VLDLR Inside ApoER2 Outside P Src/Fyn Dab1 (inside migrating neuron) But what is the process at a cellular level? - Ultimate defect is inverted layers but what causes that? - What cellular effects does Reelin have? - Not a chemoattractant - Not a “stop signal” Reelin protein shown to stimulate detachment of migrating neurons in vitro - in this case it was cells migrating to the olfactory bulb but concept same - Dab signaling shown to: (i) reduce expression of adhesion molecules (a3-integrin) (ii) promote detachment from radial glia GFP transfection can label radial units (glia and neurons) Migrating neurons fail to detach from radial glia wild-type dab1 mutants But what is the process at a cellular level? - Failure to detach from radial glia - Creates “logjam”; newly generated neurons can’t get past first ones Layers form in outside-in order, instead of inside-out order Other types of cortical defects: - Classical lissencephaly - Double cortex syndrome Classical Lissencephaly (OMIM: 607432) - Autosomal dominant - Very little folding of cortex (“smooth” cortex) - Four primitive layers instead of six - Cerebellum normal - Mental retardation, microcephaly and epilepsy Caused by mutations in LIS1 gene Double cortex syndrome (OMIM: 300067) - X-linked dominant - Cortex completely smooth and highly thickened - Four primitive layers instead of six - Severe mental retardation and epilepsy in males - Milder symptoms in heterozygous females Caused by mutations in DCX gene Staining for DCX protein highlights microtubulues QuickTime™ and a decompressor are needed to see this picture. LIS1 and DCX proteins both bind to microtubules (part of cytoskeleton that controls migration) - Required early for somal translocation (within cell’s own process) as well as later for migration along glia - Both interact with microtubules in dynamic fashion - LIS1 also interacts with VLDLR and binds phsophorylated Dab1 in response to Reelin signaling link from Dab1 to cytoskeleton Microtubuleinteracting proteins DCX Actin filamentinteracting proteins Schizophrenia as a neurodevelopmental disorder Prenatal/neonatal risk factors Early prodromal signs Distributed neuropathology - local and long-range Genes implicated - Nrg1, DISC1, DTNBP1 - have roles in neurodevelopment Summary of observed pathological changes (Frankle, 2003) Cell migration defects in schizophrenia? DISC1 - Broken by translocation in certain families with SZ - Protein binds NUDEL (which binds LIS1) - Truncated protein does not bind - Truncated form reduces neurite outgrowth when transfected into PC12 cells Mutations in DISC1 are associated with schizophrenia and other psychiatric disorders Mice with mutations in DISC1 show behavioral defects thought to be “endophenotypes” of schizophrenia: - hyperactivity - prepulse inhibition (suppression of startle response) - working memory Disrupting DISC1 function ONLY during development still results in behavioural defects => Neurodevelopmental functions of DISC1 essential for normal brain structure and function Tangential migration also very important - Especially for GABAergic (inhibitory) interneurons - Not generated in cortical ventricular zone - Generated subcortically in ganglionic eminences - Migrate long distance to cortex - Controlled by positive and negative guidance cues Interneurons generated subcortically and migrate to cortex Neuregulin-1 in control of radial migration (Flames et al., 2004, Neuron 44, 251) Nrg1 protein attracts interneurons to cortex Slice of developing mouse brain - interneurons (red) normally migrate up to cortex but will be attracted to cells expressing Nrg1 protein placed on slice (green) Mutant mice lacking Nrg1 in cortex have fewer interneurons in cortex Neuregulin-1 and ErbB4 in schizophrenia ErbB4 is receptor for secreted Nrg1 protein Nrg1 and ErbB4 both genetically associated with SZ in humans Nrg1 and ErbB4 mutants lethal in mice But heterozygotes live and show hyperactivity and other “endophenotypes” of schizophrenia e.g., working memory deficits Reversed by clozapine (no effect on wild-type activity) Summary - Cell migration is a complex process that is crucial for nervous system development in general and cortical function in particular - Severe clinical syndromes caused by mutations in a number of single genes - More subtle genetic variation in cell migration genes may contribute to multigenic disorders such as epilepsy, schizophrenia and autism. (Caveat: genes like DISC1 and Nrg1 also have nondevelopmental roles in adult) References: Olson and Walsh (2002) Curr. Opin. Genetics and Development 12, 320 Marin and Rubenstein (2001) Nat. Rev. Neurosci. 2, 780 Websites with nice movies: http://www.ipmc.cnrs.fr/~duprat/neurophysiology/video.htm http://www.rockefeller.edu/labheads/hatten/movies.html a3-integrin not downregulated in scrambler mice Integrins bind extracellular matrix molecules and mediate cell adhesion Blocking a3-integrin in scm cortical slice restores normal detachment Several types of defects observed: