* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Part Two - West Lakes GP Training
Survey
Document related concepts
Transcript
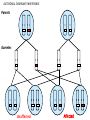
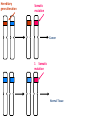
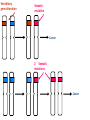
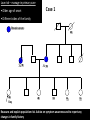
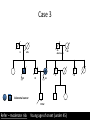
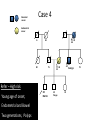
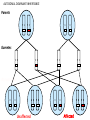
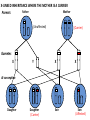
Part Two Welcome back Familial Cancer Genetics Cancer Genetics • 5-10% of all cancer clearly linked to an inherited gene alteration • If cancer seen at younger ages (before 50) possible that inherited genes increased susceptibility • Some genetic conditions increase someone’s risk of getting several different types of cancer at young age (eg. LiFraumeni syndrome, MEN 1) • Some gene alterations lead to uncontrolled cell growth: – tumour suppressor genes – oncogenes – DNA repair genes Breast Cancer • BRCA 1 & BRCA 2 testing may be available for people at high risk, but others genes known to be involved • If gene alteration found, woman at up to 80% lifetime risk of developing breast cancer • Carry risk of other cancers; ovary (BRCA 1 = 44%, BRCA 2 = 27%), and a slightly increased risk prostate, pancreas and some other cancers • Dominantly inherited through families (ie. only one copy of the altered gene needed for it to have effect) AUTOSOMAL DOMINANT INHERITANCE Parents Gametes At conception Unaffected Affected Hereditary gene alteration Somatic mutation Cancer 1 Somatic mutation Normal Tissue Hereditary gene alteration Somatic mutation Cancer 2 Somatic mutations Cancer What would indicate that a woman is at higher risk of developing breast/ovarian cancer? Relative with breast cancer before the age of 40 Relative with male breast cancer 2+ relatives with ovarian cancer Relative with bilateral breast cancer 2+ relatives on the same side of the family affected by breast cancer (especially if affected at younger ages) Breast Cancer Referral Number of Relatives Age at diagnosis Refer to Genetics 1 (first degree) > 40 years No 1 (first degree) < 40 years Yes 1 (first degree) bilateral Primary <50 years Yes 1 Male (first degree) Any age Yes ≥ 2 (one first degree) average <60 years Yes FH with Jewish ancestry Yes Strong FH on paternal side Yes Tumour associations - ovary, endometrium, prostate, bowel Yes Low risk – manage in primary care Case 1 • Older age of onset • Different sides of the family Breast cancer 65 70 46 Kay 76 49 51 53 55 Reassure and explain population risk. Advise on symptom awareness and to report any changes in family history Ovarian Cancer Number of Relatives Age at diagnosis Refer to Genetics 1 fdr no other FH of cancer Any age No 2 fdr Any age Yes 1fdr and 1sdr Any age Yes 1fdr or sdr with ovarian and 1 fdr or sdr with breast or colorectal cancer (at least 1 fdr) Any age Yes Refer – high risk • Young age onset Case 2 • Equal transmission through men • Multiple tumours in one individual • Breast and ovarian cancer 42 48 breast cancer 56 ovarian cancer Breast cancer 32 Ovarian cancer Janet Colorectal Cancer Familial Adenomatous Polyposis (FAP) Hereditary Non-Polyposis Colorectal Cancer (HNPCC). Other cancers associated with HNPCC – endometrial, stomach, ovarian Supporting Genetics Education for Health www.geneticseducation.nhs.uk Bowel Cancer Number of relatives Age at diagnosis Refer to Genetics 1 fdr >50 years No 1 fdr < 50 years Yes 2 fdr (includes both parents) Any age Yes 1fdr and 1 sdr Any age Yes 3+ relatives Any age Yes FH of known hereditary colorectal cancer syndrome (e.g. HNPCC, FAP) Any age Yes HNPCC related cancers include endometrial, gastric, ovarian, pancreatic and urothelial Case 3 73 35 died in war 60’s 73 Colorectal cancer 75 43 77 32 Peter Refer – moderate risk Young age of onset (under 45) 68 78 Case 4 Colorectal cancer Endometrial cancer 55 69 49 42 80 75 48 George Refer – High risk Young age of onset, Endometrial and Bowel Two generations, Polyps 30 Martin 39 Polyps 42 78 Referral for family history of cancer • Young age at onset, • Pattern of similar tumours on one side of the family (or multiple primaries in one individual) • Use national/local guidelines e.g. NICE familial breast cancer • Remember ethnicity e.g. – Chinese, Indian, Ashkenazi Jewish ancestry • If in doubt - Contact the Clinical Genetic Service Patient Information • Detailed information of affected family members required • Patient will receive information regarding level of risk and options • Will not necessarily mean a genetic test AUTOSOMAL DOMINANT INHERITANCE Parents Gametes At conception Unaffected Affected Familial Hypercholesterolaemia • If fulfil Simon Broome criteria, refer to specialist lipidologist • Where Genetic testing is not available, cascade testing for family members by fasting lipid profile • Children tested below 10 years • Boys have lower cholesterol during puberty Heart UK Definition using Simon Broome Register Definite Familial Hypercholesterolaemia: Total Cholesterol LDL Cholesterol Adult >7.5mmol/l >4.9mmol/l Child < 16 >6.7mmol/l >4.0mmol/l PLUS b) Tendon xanthomas in patient, or in 1st degree relative (parent, sibling, child), or in 2nd degree relative (grandparent, uncle, aunt) OR c) DNA-based evidence of an LDL receptor mutation or familial defective apo B-100 Heart UK Definition using Simon Broome Register Possible Familial Hypercholesterolaemia: Total Cholesterol LDL Cholesterol Adult >7.5mmol/l >4.9mmol/l Child < 16 >6.7mmol/l >4.0mmol/l PLUS • d) Family history of myocardial infarction: below age of 50 in 2nd degree relative or below age 60 in 1st degree relative Or • e) Family history of raised cholesterols: – >7.5 mmol/l in adult 1st or 2nd degree relative or – > 6.7 mmol/l in child or sibling under 16 X-LINKED INHERITANCE WHERE THE MOTHER IS A CARRIER Father Parents Mother (Unaffected) (Carrier) Gametes X Y X X At conception Daughter Daughter (Carrier) Son Son (Affected)