* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download H - Images
Citric acid cycle wikipedia , lookup
Deoxyribozyme wikipedia , lookup
Cell-penetrating peptide wikipedia , lookup
Artificial gene synthesis wikipedia , lookup
Vectors in gene therapy wikipedia , lookup
Nucleic acid analogue wikipedia , lookup
Fatty acid synthesis wikipedia , lookup
Biosynthesis wikipedia , lookup
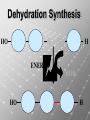
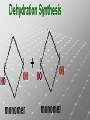
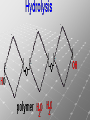
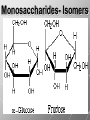
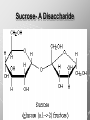
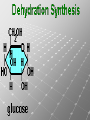
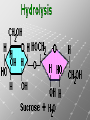
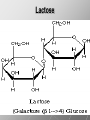
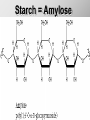
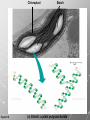
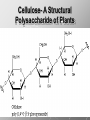
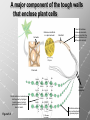
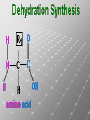
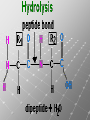
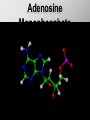
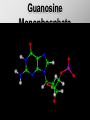
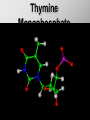
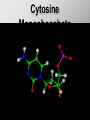
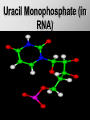
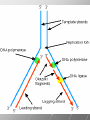
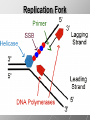
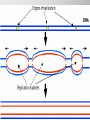
Ch. 4- Carbon and the Molecular Diversity of Life Structure & Ch. 5- Function of Macromolecules Chapters 4 and 5 The Uniqueness of Carbon Requires 4 electrons to fill its outer shell. Will form tetrahedral molecules with other atoms. Has equidistant bond angles of 109.5°. Will readily form single, double and triple covalent bonds. Carbon forms a variety of chained and ringed organic compounds. Carbon is the backbone for many organic compounds. Carbon in a Tetrahedron! Hydrocarbons: Are molecules consisting of only carbon and hydrogen. Are found in many of a cell’s organic Fat droplets (stained red) molecules. 100 µm Figure 4.6 A, B (a) A fat molecule (b) Mammalian adipose cells Functional groups are the parts of molecules involved in chemical reactions Functional groups Are the chemically reactive groups of atoms within an organic molecule. Six functional groups are important in the chemistry of life Hydroxyl Carbonyl Carboxyl Amino Sulfhydryl Phosphate P. 64 Functional groups give organic molecules distinctive chemical properties Estradiol CH3 OH HO Female lion CH3 OH CH3 O Figure 4.9 Male lion Testosterone Organic Compounds Four major groups: 1. Carbohydrates 2. Lipids 3. Proteins 4. Nucleic Acids Differ in their functional groups Organic Compounds Some organic compounds are small with one or a few functional groupsmonomers. (Ex. Glucose = monosaccharide). Other organic compounds are made from linking several simple monomers together in complex chains- polymers (1000’s of glucose monomers = starch, polysaccharide). Monomers Simple Polymers Complex Monosaccharides Polysaccharides Glycerol, Fatty Acids Lipids, Fats Amino Acids Proteins Nucleotides Nucleic Acids Building Macromolecules All polymers are formed by making covalent bonds between two monomers. The –OH group from one monomer is removed and the –H from the other is removed – Dehydration Synthesis H2O is removed which requires energy. Dehydration Synthesis HO H ENERGY HO HO H HOH H Dehydration Synthesis When polymers are built from smaller monomers- anabolic reactions (synthesizing). Requires energy. These reactions require the reactants to be held close together and chemical bonds to be stressed and broken-catalysis. Catalysis is caused by enzymes. Hydrolysis Reactions Cells may also disassemble polymers into monomers- catabolic reactions (breakdown). A molecule of H2O is added and split; a H is added to one monomer and the OH is added to the other-hydrolysis (water splitting). Catabolic reactions release the energy stored in the bonds of the monomers. Carbohydrates Contain C, H, O atoms (CH2O)n Functions: Main source of energy- for immediate use or for energy storage, Used for structure- on surfaces of cell membranes (bacteria, eukaryotes), or support cell walls (plants). Three Types of Carbohydrates 1. Monosaccharides- “mono”- single; simple sugars that are made of 3-6 C’s in a chain or ring. Ex. C6H12O6 , Glucose, most abundant monosaccharide Straight Chain or Rings Monosaccharides- Isomers Three types of isomers are: Structural Geometric Enantiomers (a) Structural isomers H H H H H H C C C C C H H H H H X (b) Geometric isomers H H H C H C NH2 CH3 Figure 4.7 A-C X CO2H C H H X H CO2H c) Enantiomers H C C C H C H H H C C H X C H H H C H H NH2 CH3 H Enantiomers: Are important in the pharmaceutical industry. Figure 4.8 L-Dopa D-Dopa (effective against Parkinson’s disease) (biologically inactive) Isomers Structural Isomersmonosaccharides with the same empirical formula but different structures. Ex. Glucose and Fructose Stereoisomers – monosaccharides that have the same empirical formula but they have functional groups as mirror images of each other. Ex. Glucose and Galactose Monosaccharides of Nucleic Acids Other Monosaccharides Fructose- commonly found in fruit. Galactose- found in milk. Ribose- found in RNA. Deoxyribose- found in DNA. Monosaccharides Most offer a number C-H bonds as potential chemical energy. May also be used as monomers to build more complex polymers for energy storage or structural molecules. 2. Disaccharides Are two monosaccharides that form a glycosidic bond by removing a H2O molecule. Glucose + Fructose-->Sucrose (table sugar) Sucrose- A Disaccharide Disaccharides Monosaccharides (glucose) is often converted into a disaccharide before being transported around an organism’s body. Unable to be used in this form until it arrives at a tissue. Plants transport glucose as sucrose. (sugar cane) Lactose Lactose Mammals use lactose to transport glucose to infant. Adults usually lack the enzyme, lactase, which breaks down lactose glucose + galactose. Other Disaccharides Sucrose (Table Sugar)- Glucose + Fructose Lactose (Milk Sugar)- Glucose + Galactose Maltose (Breakdown from Starch)Glucose + Glucose 3. Polysaccharides Formed when monosaccharides are linked in chains by glycosidic bonds. They are polymers- long chains of monomers (building blocks). Polymer = polysaccharide, Monomers = monsaccharides Polysaccharides Two Basic Functions1. Storage Polysaccharides: May store 1000’s of monomers for energy. Usually stored in special storage structures. 2. Structural: May form structural parts of cells and/or tissues. Starch = Amylose Chloroplast Starch 1 m Amylose Figure 5.6 Amylopectin (a) Starch: a plant polysaccharide Plant Storage- Starch Amylose- hundreds of glucose molecules in a long, unbranched chain. The glycosidic bond is between the 1C-4C. The chains coil in water and don’t form H bonds, therefore not very soluble in H2O. Only 20% of starch in potatoes is amylose. 80% is amylopectin- short and branched glucose chains. Is cross-linked. Starch Storage Plants use special tissues called tubers. Also stored in bulbs of perennials. Glycogen: Consists of glucose monomers. Is the major storage form of glucose in animals. Mitochondria Giycogen granules 0.5 m Glycogen Figure 5.6 (b) Glycogen: an animal polysaccharide Animal Storage- Glycogen Insoluble, branched amylose chains. Longer and more branched than starch. Stored in liver and skeletal muscle. Not transported in blood. Starch Cellulose Cellulose has different glycosidic linkages than starch. H O C CH2OH H 4 O H OH H HO HO C H H C OH H C OH H C OH OH OH H C H H CH2OH OH glucose H O H OH 4 HO OH 1 H H H OH glucose (a) and glucose ring structures CH2OH CH2OH O HO O 4 1 OH O 4 O 1 OH OH OH O O 1 OH CH2OH CH2OH O 4 1 OH O OH OH (b) Starch: 1– 4 linkage of glucose monomers OH CH2OH O HO O OH 1 OH O O OH CH2OH OH O O OH Figure 5.7 A–C 4 OH CH2OH O OH (c) Cellulose: 1– 4 linkage of glucose monomers CH2OH OH Structural Polysaccharides Cellulose- a chain of glucose molecules in which the monomers alternate positions. Similar to amylose but not recognized by the same enzymes. A water-tight, structural molecule. Plant cell walls Cellulose- A Structural Polysaccharide of Plants A major component of the tough walls that enclose plant cells Cellulose microfibrils in a plant cell wall Cell walls Microfibril About 80 cellulose molecules associate to form a microfibril, the main architectural unit of the plant cell wall. 0.5 m Plant cells Parallel cellulose molecules are held together by hydrogen bonds between hydroxyl groups attached to carbon atoms 3 and 6. Figure 5.8 OH CH2OH OH CH2OH O O O O OH OH OH OH O O O O O O CH OH OH CH2OH 2 H CH2OH OH CH2OH OH O O O O OH OH OH OH O O O O O O CH OH OH CH2OH 2 H CH2OH OH OH CH2OH O O O O OH OH OH OH O O O O O O CH OH OH CH2OH 2 H Glucose monomer Cellulose molecules A cellulose molecule is an unbranched glucose polymer. Cellulose is difficult to digest: Cows have microbes in their stomachs to facilitate this process (relationship?). Figure 5.9 Polysaccharides and Clean Hair! Chitin, another important structural polysaccharide Is found in the exoskeleton of arthropods. Can be used as surgical thread. CH2O H O OH H H OH H OH H H NH C O CH3 (a) The structure of the chitin monomer. Figure 5.10 A–C (b) Chitin forms the exoskeleton of arthropods. This cicada is molting, shedding its old exoskeleton and emerging in adult form. (c) Chitin is used to make a strong and flexible surgical thread that decomposes after the wound or incision heals. 3. Chitin Structural polysaccharide of Arthropods (insects and crustaceans) and fungi. Modified form of cellulose; has an added nitrogen group to each glucose unit. Hard, flexible, and water-tight. Few organisms can digest. Exoskeleton of Arthropods. Polysaccharide? Lipids Have C, H, O, but more H and less O than carbohydrates. Four Kinds: Fats, Phospholipids, Terpenes, Steroids Functions of Lipids: 1. May form cell membranes. 2. Used for longterm energy storage. Does the Cell Make Me Look Fat? Stored Fat Nucleus 1. Fats A Fat = Glycerol + 1 or more Fatty Acids Glycerol- a 3 carbon alcohol. Fatty Acid- HO - C – R O Where R = hydrocarbon chain. Synthesis of a Fat Dehydration Synthesis removing H2O and forming an ester bond between the OH of glycerol and the carboxyl group of a fatty acid. Fats: are constructed from two types of smaller molecules, a single glycerol and usually three fatty acids. H H C O C OH HO H C OH H C OH H C H H C H H C H H C H H C H H C H H C H H C H H C H H C H H C H H C H H C H H C H Fatty acid (palmitic acid) H Glycerol (a) Dehydration reaction in the synthesis of a fat Ester linkage O H H C O C H C H O H C O C O H C H Figure 5.11 O C H C H H C H C H H H C H C H H H C H H C H H C H H C H C H H H C H H C H H C H H C H H C H H C H H C H H C H H C H H C H (b) Fat molecule (triacylglycerol) H C H H C H H C H H C H H C H H C H H C H H C H H C H H C H H C H H C H H C H H H C C H H H C H H C H H C H H C H H C H H C H H C H H H C H H H C H H H C H H A Glycerol Three Fatty Acids Types of Fatty Acids Saturated Fatty Acids- single-bonded hydrocarbon chains completely filled with H. Solids at Room Temp. Usually from animals. Ex. Lard Unsaturated Fatty Acids- usually contain a double bond within the hydrocarbon chain, at least one carbon may bond with a H. Liquids at room temp. Derived from plants. Ex. Veg. Oil Saturated Fatty Acids: -have the maximum number of hydrogen atoms possible. -have no double bonds. Stearic acid Figure 5.12 (a) Saturated fat and fatty acid Unsaturated Fatty Acids: Have one or more double bonds. Oleic acid Figure 5.12 (b) Unsaturated fat and fatty acid cis double bond causes bending So what is Peanut Butter? If it comes from a plant, it should be a(n)…. Unsaturated Fat! But it’s a solid! It is a hydrogenated fat! The Importance of Fats Efficient energy-storage molecules because of high # of C-H bonds. More than twice the # in carbs. Convert unused glucose into starch or fats. Fats = 9 kcal/g; Carb = 4 kcal/g Energy in Saturated fats > Unsaturated fats 2. Phospholipids Substitute a phosphate (PO4) for one fatty acid in a triglyceride. The (PO4) group is polar (charged). The polar ends interact with each otherHydrophilic The nonpolar (uncharged) hydrocarbon chains interact- Hydrophobic Found in all cell membranes. Phospholipid structure: Consists of a hydrophilic “head” and hydrophobic “tails.” + N(CH ) CH2 3 3 Choline CH2 O O P O– Phosphate O CH2 CH O O C O C CH2 Glycerol O Fatty acids Hydrophilic head Hydrophobic tails Figure 5.13 (a) Structural formula (b) Space-filling model (c) Phospholipid symbol The structure of phospholipids: Results in a bilayer arrangement found in cell membranes. WATER Hydrophilic heads WATER Hydrophobic tails Figure 5.14 3. Terpenes Long chain lipids; many CH bonds. Many photosynthetic pigments are terpenes. Examples are the cholorophyls and carotenes. Rubber is a terpene. 4. Steroids and other ringed lipids Four-ringed Structures. Ex. Cholesterol, hormones, vitamin D Animal cell membranes contain cholesterol, most bacteria do not. Lipids are Effective Barriers At the cellular level – phospholipids. A waxy covering on the epidermis of plants – cutin. Coating of bird wings – oils. Other Fats provide a way of absorbing and storing fat-soluble vitamins A, D, E, K. DDT: a fat-soluble poison and the osprey Genetics Basic unit of heredity- Gene- a linear sequence of nucleotides of DNA. Genotype- genetic make-up of organism; its potential characteristics. Phenotype- the observable physical traits of an organism. The Phenotype is the organism’s physical expression of its Genotype. Eukaryotes are Diploid Locus - is a gene’s location on the chromosome. Allele- an alternative form of a gene at a specific locus. Eukaryotes have pairs of identical chromosomes- diploid. May have two alleles of a gene. Prokaryotes are not diploid. Mutation A permanent change in the sequence of nucleotides mutation. Mutations change the information of that gene. DNA- function is to store and transfer information. How is DNA Accurately Transferred? DNA serves as a template for its own replication; an exact pattern. How, you ask? By base pairing. What is a Nucleotide? Subunits of DNA/RNA are Nucleotides = nitrogenous base + deoxy- or ribose sugar (5 carbons) + PO4 Purines: Adenine and Guanine Pyrimidines: Cytosine, Thymine, Uracil Monosaccharides of Nucleic Acids Adenosine Monophosphate Base = adenine In DNA, sugar = deoxyribose (In RNA, sugar = ribose) A phosphate group, PO4 The Nucleotide = AMP Adenosine Monophosphate Guanosine Monophosphate Thymine Monophosphate Cytosine Monophosphate Uracil Monophosphate (in RNA) Base Pairing Rules In DNA, A=T C G In RNA, A=U C G H-Bonding Between Bases Characteristics of DNA Chains of nucleotides linked together by phosphodiester bonds. Carbon 5 of deoxyribose is attached to PO4. Carbon 3 of deoxyribose is a OH- free to attach to the next nucleotide. Double helix is held together by Hbonding. Double Helix DNA is antiparallel: 5’PO4 ------------------------3’OH 3’OH ------------------------5’PO4 DNA Replication Begins at a specific location on the circular bacterial chromosome-origin (OriC). Occurs in two directions at the same time-two moving replication forkspoints where the two strands separate to allow replication of DNA. Replication Fork DNA Replication Prokaryotes Eukaryotes single, circular multiple, linear chromosome. chromosomes. one single origin several origins on on the each chromosome. chromosome. rate of over 1,000 rate of about 50nt/second. 100 nt/second