* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Acid-Base_Handling
Single-unit recording wikipedia , lookup
Stimulus (physiology) wikipedia , lookup
Resting potential wikipedia , lookup
12-Hydroxyeicosatetraenoic acid wikipedia , lookup
Common raven physiology wikipedia , lookup
Homeostasis wikipedia , lookup
Alveolar macrophage wikipedia , lookup
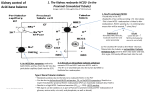
Renal Acid-Base Handling Introduction • [H+] is maintained within narrow limits • Normal extracellular [H+] ≈ 40 nanomol/L (one-millionth the mmol/L concentrations of Na+, K+, Cl-, HCO3-) • Regulation of [H+] at this low level is essential for normal cellular (protein) fxn – Increase in [H+] change charge, shape and function of proteins 3 Basic Steps of H+ Regulation • Chemical buffering by extracellular and intracellular buffers • Control of partial pressure of CO2 in the blood by alterations in alveolar ventilation • Control of plasma [HCO3-] by changes in renal H+ excretion Buffers • Take up or release H+ ions to maintain a stable [H+] • HPO42- (base) + H+ ⇄ H2PO4- (acid) • HCO3- (base) + H+ ⇄ H2CO3 (acid) Henderson-Hasselbalch Equation • Ka (dissociation constant) = • [H+] = Ka [H+] [A-] [HA] [HA] [A-] • -log [H+] = -log Ka - log [HA] • pH = pKa + log [A-] [A-] [HA] • pH = 6.10 + log [HCO3-]/0.03 Pco2 • H+ + HCO3- ⇄ H2CO3 ⇄ H20 + CO2 Bicarbonate Buffer System • The major physiologic buffer system • [HCO3-] and Pco2 regulated independently – [HCO3-] regulated by renal H+ excretion – Pco2 regulated by changes in alveolar ventilation • As H+ are buffered by HCO3, elevation in Pco2 is prevented by increase in alveolar ventilation, thus enhancing effectiveness of HCO3 buffering • Capable of removing large quantity of H+ due to large amount of HCO3 in the body Buffering During Metabolism of SulfurContaining Amino Acids Diet ECF 2H+ 2 CO2 + 2 H2O 2 HCO3- Sulfur-AA SO42- 2 HCO3Glutamine SO422 NH4+ 2 NH4+ Kidney Urine •Acid balance is achieved when SO42- are excreted in the urine with NH4+ because HCO3- is generated in the process Buffering During Metabolism of Organic Phosphates Diet ECF H+ HCO3- CO2 + H2O RNA-PHCO3HPO42H2PO4- Urine CO2 + H2O H+ Kidney Base Balance During Metabolism of Organic Anions Diet ECF liver HCO3- H+ CO2 K+ + OA- Glucose OAK+ OAKidney Urine liver Acid-Base Balance Acid-Base Balance Production H+ Removal H+ Acid Balance Base Balance Diet 2H+ + SO42- Diet 3K+ + 3HCO3- 2H+ + HCO3- 2CO2 + 2H2O Glucose 3H+ + Citrate3- Add “new” HCO3- 2NH4 + SO4 2- Removal HCO3- Excrete OA Urine + Production HCO3- 3K+ + Citrate3- Alveolar Ventilation • Main physiologic stimuli to respiration – Pco2 • Chemoreceptors in respiratory center in brainstem respond to CO2-induced ∆ cerebral interstitial pH – Po2 • Peripheral chemoreceptors in the carotid bodies Sequential Response to H+ Load Extracellular buffering by HCO3- Intracellular and bone buffering Immediate 2-4 hours Respiratory buffering by Pco2 renal H+ excretion Minutes-Hours Hours to Days Renal H+ Excretion: Basic Principles • Achieved by H+ secretion – Na+/H+ exchange: proximal tubules and thick ascending limb of the LOH – H+-ATPase: collecting tubules • Acid load cannot be excreted as free H+ ions – Urinary [H+] is extremely low (< 0.05 mEq/L) in the physiologic pH range Renal H+ Excretion: Basic Principles • Acid load cannot be excreted unless virtually all of the filtered HCO3- has been reabsorbed • Secreted H+ ions bind to: – Filtered buffers (HPO42-, creatinine) – NH3 to form NH4+ • Rate of NH4+ generation in the proximal tubules varies according to physiologic needs Renal H+ Excretion: Basic Principles • Extracellular pH is the primary physiologic regulator of net acid excretion – Other factors include: • Effective circulating volume • Aldosterone • Plasma [K+] 2 Basic Steps of Renal H+ Excretion • Reabsorption of the filtered HCO3• Excretion of 50-100 mEq of H+ produced per day (daily acid load on a typical Western diet) Reabsorption of Filtered HCO3• Loss of filtered HCO3- = addition of H+ • Virtually all of the filtered HCO3- must be reabsorbed – Normal person reabsorbs about 4300 mEq of HCO3- per day (GFR 180 L/day x 24mEq/L HCO3- ) Renal H+ Secretion • Secreted H+ ions are generated within tubular cells from dissociation of H2O • OH- ions combine with CO2 to form HCO3-, catalyzed by intracellular carbonic anhydrase – HCO3- is absorbed across basolateral membrane • Secretion of one H+ ion in the urine = generation of one HCO3- in the plasma Renal H+ Secretion • If secreted H+ combines with filtered HCO3- , the result is HCO3- reabsorption thus preventing HCO3- loss in the urine • If secreted H+ combines with HPO42- or NH3, a new HCO3- is added to the plasma (replaces the HCO3- lost in buffering the daily H+ load) Net Acid Excretion Net Acid Excretion (NAE) = titratable acid + NH4+ - urinary HCO3- quantity not replenishable (HPO42-, Cr) excretion can be increased Titratable acid represents the amount of alkali that is required to titrate the urine pH back to the plasma pH (7.4) Proximal Acidification • Proximal tubules reabsorb 90% of filtered HCO3• Primary step is secretion of H+ by Na+-H+ exchanger in luminal membrane – Energy indirectly provided by Na+/K+ ATPase in basolateral membrane • HCO3- returned to systemic circulation by Na+/3 HCO3- cotransporter • Carbonic anhydrase plays central role Proximal Acidification UpToDate, 2009 Distal Acidification • H+ secretion in distal nephron occurs in type A intercalated cells in the cortical collecting tubule and in the cells of the medullary colllecting tubule • H+ secretion is mediated by active luminal secretory pumps – H+-ATPase – H+/K+ ATPase Distal Acidification • H+ secretion by intercalated cells is indirectly influenced by Na+ reabsorption in the adjacent principal cells – Na+ absorption makes the lumen relatively electronegative, thus promoting H+ secretion • HCO3- reabsorption across basolateral membrane is mediated by Cl-/ HCO3- exchanger Type A Intercalated Cell UpToDate, 2009 Type B Intercalated Cell UpToDate, 2009 Type A vs B Intercalated Cells Ammonium Generation and Excretion Glutamine 2-Oxoglutarate2- + 2 NH4+ Liver 2 NH4+ Urea 2HCO3- to body 2 NH4+ in urine 2 HCO3- Ammonium Generation and Excretion Exogenous Endogenous Proteins 2HCO3- Methionine + Glutamine 2H+ + SO422 CO2 + 2H2O 2NH4+ H+ NH4+ + NH3 2HCO32NH4+ + SO42- Ammonium Generation and Excretion UpToDate, 2009 Medullary Ammonium Recycling Fluid, Electrolyte and Acid-Base Physiology, 2010 Ammonium Generation and Excretion Comprehensive Pediatric Nephrology, 2008 Regulation of Renal H+ Excretion • Extracellular pH • Effective circulating volume – Renin-angiotensin-aldosterone system – Chloride depletion • Plasma potassium Extracellular pH Is Major Regulator of Renal H+ Excretion • NAE varies inversely with extracellular pH • Acidemia⇑prox and distal acidification – Proximal tubule • • • • ⇑luminal Na+/H+ exchange ⇑luminal H+-ATPase activity ⇑Na+/3HCO3- activity in basolateral membrane ⇑NH4 production from glutamine – Collecting tubule • ⇑luminal H+-ATPase activity in intercalated cells • Alkalemia⇓prox HCO3- reabsorption and ⇑HCO3secretion in CCD Effective Circulating Volume • Hypovolemia activates RAAS system, causing HCO3- reabsorption – Angiotensin II • ⇑luminal Na+/H+ exchange in proximal tubule • ⇑basolateral Na+/3HCO3- activity in proximal tubule – Aldosterone • ⇑luminal H+-ATPase activity in collecting tubule • ⇑basolateral Cl-HCO3- activity in collecting tubule • ⇑Na+ absorption in principal cells in cortical collecting tubule, resulting in net H+ secretion Effective Circulating Volume • Hypochloremia commonly occurs in metabolic alkalosis – Low filtered [Cl-] increases H+ secretion • Cl- is passively cosecreted with H+ secretion via H+ATPase to maintain electroneutrality thus ability to secrete H+ is enhanced with low tubular fluid [Cl-] • In setting of low tubular fluid [Cl-], Na+ reabsorption must be accompanied by H+ or K+ secretion in CCD Hypochloremia Decreases HCO3Secretion Type B Intercalated Cell • Energy for luminal Cl/HCO3- exchange is provided by favorable inward gradient for Cl• Low tubular [Cl-] ⇓gradient thus less HCO3secreted UpToDate, 2009 Plasma K+ Influences Renal H+ Secretion Hypokalemia Cell ECF K+ Changes in K+ balance lead to transcellular cation shifts that affect intracellular [H+] Hypokalemia leads to low intracellular pH H+ Na+ Intratubular Acidosis Increases H+ Excretion in Hypokalemia • ⇑H+ secretion in proximal tubule – ⇑luminal Na+/H+ exchange – ⇑basolateral Na+/3HCO3- activity • ⇑NH4 generation from glutamine in proximal tubule • ⇑H+ secretion in distal nephron – ⇑luminal H+/K+ ATPase, resulting in H+ secretion and K+ absorption Renal Acification: Summary Molecular and Genetic Basis of Renal Disease, 2007 References • Rose BD, Post TW: Clinical Physiology of AcidBase and Electrolyte Disorders. New York, McGraw-Hill, 2001, pp 299-371. • Bidani A, Tuazon DM, Heming TA: Regulation of Whole Body Acid-Base Balance. In DuBose TD, Hamm LL (eds): Acid-Base and Electrolyte Disorders. Philadelphia, Saunders, 2002, pp 121. References • Alpern RJ, Hamm LL: Urinary Acidification. In DuBose TD, Hamm LL (eds): Acid-Base and Electrolyte Disorders. Philadelphia, Saunders, 2002, pp 23-40. • Halperin ML, Goldstein MB, Kamel KS: Fluid, Electrolyte and Acid-Base Physiology. Saunders, 2010, pp 3-29. References • UpToDate, 2009 • Mount DB, Pollak MR: Molecular and Genetic Basis of Renal Disease: A Companion to Brenner and Rector’s The Kidney. Saunders, 2007. • Geary D, Schaefer F: Comprehensive Pediatric Nephrology. Mosby, 2008. Distal Acidification Comprehensive Pediatric Nephrology, 2008 Chronic Metabolic Acidosis and Respiratory Compensation Clinical state Arterial pH [HCO3-], mEq/L Pco2, mmHg Baseline 7.40 24 40 7.29 19 40 Acute 7.37 19 34 Chronic 7.29 16 34 Metabolic acidosis No compensation Compensation Medullary Transfer of Ammonium Fluid, Electrolyte and Acid-Base Physiology, 2010