* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Glycerolipids and Glycerophospholipids
Butyric acid wikipedia , lookup
Oligonucleotide synthesis wikipedia , lookup
Proteolysis wikipedia , lookup
Artificial gene synthesis wikipedia , lookup
Peptide synthesis wikipedia , lookup
Lipid signaling wikipedia , lookup
Amino acid synthesis wikipedia , lookup
Fatty acid synthesis wikipedia , lookup
Biosynthesis wikipedia , lookup
Glyceroneogenesis wikipedia , lookup
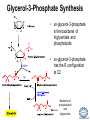
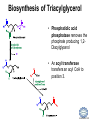
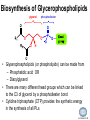
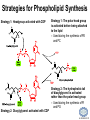
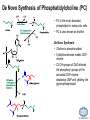
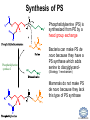
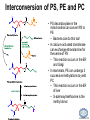
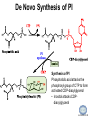
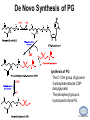
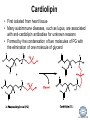
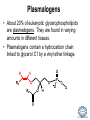
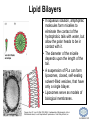
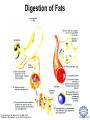
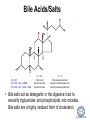
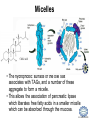
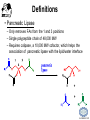
LIPID MAPS Lipid Metabolomics Tutorial Glycerolipids and Glycerophospholipids Professor Edward A. Dennis Department of Chemistry and Biochemistry Department of Pharmacology, School of Medicine University of California, San Diego Copyright/attribution notice: You are free to copy, distribute, adapt and transmit this tutorial or individual slides (without alteration) for academic, non-profit and non-commercial purposes. Attribution: Edward A. Dennis (2010) “LIPID MAPS Lipid Metabolomics Tutorial” www.lipidmaps.org E.A. DENNIS 2010 © Types of Biological Lipids • Lipids derived from ketoacyl units – Fatty acids (inc. Prostaglandins and wax esters) – Glycerolipids – Glycerophospholipids – Sphingolipids – Saccharolipids – Polyketides • Lipids derived from isoprene units – Sterols – Prenols (inc. terpenes and Fat Soluble Vitamins) • Mixtures – Lipoproteins E.A. DENNIS 2010 © Definitions Glycerophospholipid = an amphipathic lipid in which two fatty acyl groups are attached to a glycerol-3-phosphate whose phosphate group is linked to a polar group X=head group Usually a saturated FA Phosphatidic acid = the simplest glycerophospholipid -the precursor to other phospholipids and to triacylglycerols. Also called diacylglycerol-3-phosphate. Usually an unsaturated FA Triacylglycerol = a lipid in which three fatty acids are esterified by a glycerol backbone. It is the major form of energy storage in humans. Also called a triglyceride. E.A. DENNIS 2010 © Glycerol-3-Phosphate Synthesis • sn-glycerol-3-phosphate is the backbone of triglycerides and phospholipids • sn-glycerol-3-phosphate has the R configuration at C2 Backbone of phospholipids and triglycerides E.A. DENNIS 2010 © Biosynthesis of Phosphatidic Acid • Precursors – Fatty acids – sn-glycerol-3-phosphate • sn-glycerol-3-phosphate is produced from the – Reduction of DHAP by glycerol phosphate dehydrogenase OR – Phosphorylation of glycerol by glycerol kinase and ATP • Acyl transferases perform two successive esterifications with fatty acyl Co A to generate phosphatidic acid E.A. DENNIS 2010 © Biosynthesis of Triacylglycerol • Phosphatidic acid phosphatase removes the phosphate producing 1,2Diacylglycerol • An acyl transferase transfers an acyl CoA to position 3. E.A. DENNIS 2010 © Biosynthesis of Glycerophospholipids glycerol phosphodiester • Glycerophospholipids (or phospholipids) can be made from – Phosphatidic acid OR – Diacylglycerol • There are many different head groups which can be linked to the C3 of glycerol by a phosphodiester bond • Cytidine triphosphate (CTP) provides the synthetic energy in the synthesis of all PLs E.A. DENNIS 2010 © Strategies for Phospholipid Synthesis Strategy 1: Headgroup activated with CDP Strategy 1: The polar head group is activated before being attached to the lipid – Used during the synthesis of PE and PC Strategy 2: The hydrophobic tail of diacylglycerol is activated rather than the polar head group – Used during the synthesis of PI and PG Strategy 2: Diacylglycerol activated with CDP E.A. DENNIS 2010 © De Novo Synthesis of Phosphatidylcholine (PC) • PC is the most abundant phospholipid in eukaryotic cells • PC is also known as lecithin De Novo Synthesis • Choline is phosphorylated • Cytidyltransferase makes CDPcholine • C3 OH groups of DAG attacks the phosphoryl groups of the activated CDP-choline displacing CMP and yielding the glycerophospholipid E.A. DENNIS 2010 © De Novo Synthesis of PE • PE is the second most abundant phospholipid in eukaryotic cells De Novo Synthesis • Ethanolamine is phosphorylated • Cytidyltransferase makes CDP-ethanolamine • C3 OH groups of DAG attacks the phosphoryl groups of the activated CDP-ethanolamine or displacing CMP and yielding the glycerophospholipid E.A. DENNIS 2010 © Synthesis of PS Phosphatidylserine (PS) is synthesized from PE by a head group exchange Phosphatidylserine synthase 2 Bacteria can make PS de novo because they have a PS synthase which adds serine to diacylglycerol(Strategy 1 mechanism) Mammals do not make PS de novo because they lack this type of PS synthase E.A. DENNIS 2010 © Interconversion of PS, PE and PC • PS decarboxylase in the mitochondria can convert PS to PE – Bacteria can do this too! • A calcium-activated transferase can exchange ethanolamine for the serine of PS – This reaction occurs in the ER and Golgi • In mammals, PE can undergo 3 successive methylations to yield PC – This reaction occurs in the ER of liver – S-adenosylmethionine is the methyl donor E.A. DENNIS 2010 © De Novo Synthesis of PI Synthesis of PI Phosphatidic acid attacks the phosphoryl group of CTP to form activated CDP-diacylglycerol • Inositol attacks CDPdiacylglycerol E.A. DENNIS 2010 © Phosphatidylinositol Phosphorylation These OH groups can also be esterified with PO32Phosphatidylinositol (PI) • PI can be phosphorylated to different degrees • PIP2 = phosphatidylinositol 4,5-bisphosphate is very important in signal transduction – When a receptor G protein is activated it can mediate the cleavage of PIP2 to DG and IP3 – DG activates protein kinase C which adds phosphates to certain proteins – IP3 mobilizes intracellular Ca and activates certain cell processes E.A. DENNIS 2010 © De Novo Synthesis of PG Synthesis of PG • The C1 OH group of glycerol3-phosphate attacks CDPdiacylglycerol • The phosphoryl group is hydrolyzed to form PG E.A. DENNIS 2010 © Cardiolipin • First isolated from heart tissue • Many autoimmune diseases, such as lupus, are associated with anti-cardiolipin antibodies for unknown reasons • Formed by the condensation of two molecules of PG with the elimination of one molecule of glycerol E.A. DENNIS 2010 © Plasmalogens • About 20% of eukaryotic glycerophospholipids are plasmalogens. They are found in varying amounts in different tissues. • Plasmalogens contain a hydrocarbon chain linked to glycerol C1 by a vinyl ether linkage. E.A. DENNIS 2010 © Major Components of Membranes • Proteins – – – – Channels & Pumps Structural proteins Receptors Reaction enzymes • Lipids – Sterols (Cholesterol) – Glycerophospholipids – Glycerolipids (neutral lipids) – “Surfactant” – Sphingolipids E.A. DENNIS 2010 © Lipid Bilayers • In aqueous solution, amphiphilic molecules form micelles to eliminate the contact of the hydrophobic tails with water, but allow the polar heads to be in contact with it. • The diameter of the micelle depends upon the length of the tail. • A suspension of PLs can form liposomes, closed, self-sealing solvent-filled vesicles, that have only a single bilayer. • Liposomes serve as models of biological membranes. Figures: Voet, D, Voet JG, Pratt CW (2006), Fundamentals of Biochemistry: Lif e at the Molecular Level, 2nd ed. Reprinted with permission of John Wiley & Sons, Inc. E.A. DENNIS 2010 © Movement in Bilayers Key Principle: Things on one side tend to stay on that side... …unless specifically moved by a carrier protein called a “flippase.” Some Implications: • Lipid populations on each side can be different • Receptors aim out • Embedded/anchored enzymes localize reactions to only one side • Ion pumps move the same ions the same direction • Etc. Figure: Voet, D, Voet JG, Pratt CW (2006), Fundamentals of Biochemistry: Lif e at the Molecular Level, 2nd ed. Reprinted with permission of John Wiley & Sons, Inc. E.A. DENNIS 2010 © Membrane-Air Interface • Membrane-Water – Low surface tension – Membrane is “Compliant” • large change in volume for a small change in pressure • Membrane-Air (lungs) – High surface tension – Membrane is “Resistant” • small volume change for a large pressure change Comparative inflation of lung tissue when filled with liquid (low surface tension) vs. with air (high surface tension). Figure: West JB, Respiratory Physiology – The Essentials , 2000. E.A. DENNIS 2010 © DPPC = Surfactant • Dipalmitoyl phosphatidylcholine (DPPC) • Main function: reduces surface tension at the alveoli-air interface (increases compliance) E.A. DENNIS 2010 © Diagnostics: the L/S Ratio • Phosphatidylcholine (PC) is produced by type II alveolar epithelial cells in the fetal lung. During gestation it is found in the amniotic fluid. PC 22 18 Amniotic Fluid14 Concentration (mg/dl) 10 • Sphingomyelin remains at a low level throughout gestation. It can be used as a baseline for comparison • The L/S ratio is close to 1:1 until the 30-35th week of gestation. At this time, lecithin content increases dramatically attaining a ratio of greater than 2:1. This indicates pulmonary maturity. Sphingomyelin 6 2 18 22 26 30 34 38 Term Gestation (weeks) • This test is very accurate in determining the absence of RDS; but patients with a ratio <2:1 may not have RDS. E.A. DENNIS 2010 © Digestion of Fats Figure: Lehninger AL, Nelson DL, Cox MM (1993), Principles of Biochemistry, 2nd ed. Worth Publishers, Inc. E.A. DENNIS 2010 © Bile Acids/Salts R2 = OH R2 = NH – CH2 – COOH R2 = NH – CH 2 – CH2 – SO3H R1 = OH Cholic acid Glycocholic acid Taurocholic acid R1 = H Chenodeoxycholic acid Glycochenodeoxycholic acid Taurochenodeoxycholic acid • Bile salts act as detergents in the digestive tract to emulsify triglycerides and phospholipids into micelles. Bile salts are a highly oxidized form of cholesterol. E.A. DENNIS 2010 © Micelles • The hydrophobic surface of the bile salt associates with TAGs, and a number of these aggregate to form a micelle. • This allows the association of pancreatic lipase which liberates free fatty acids in a smaller micelle which can be absorbed through the mucosa. E.A. DENNIS 2010 © Definitions • Lipases or Acyl Hydrolases – Triacylglycerol Lipase – the general term. – Lingual Lipase- found in saliva for pre-digestion. – Pancreatic Lipase- produced by the pancreas for digestion. – Lipoprotein lipase- found on capillary endothelial cells. It hydrolyzes TAG in chylomicrons and VLDLs. – Hormone Sensitive Lipase- generates FAs when energy is needed. Found in adipose tissue. E.A. DENNIS 2010 © Definitions • Pancreatic Lipase – Only removes FAs from the 1 and 3 positions – Single polypeptide chain of 48,000 MW – Requires colipase, a 10,000 MW cofactor, which helps the association of pancreatic lipase with the lipid/water interface E.A. DENNIS 2010 © Phospholipase Sites of Action 1-palmitoyl, 2-oleoyl-phosphatidylinositol- 4’,5’ bisphosphate (PIP2) E.A. DENNIS 2010 © More Definitions • Pancreatic Phospholipase A2 – Removes FAs from the 2 position – 124 aa single polypeptide chain of 14,000 MW – Produced as a zymogen. Trypsin hydrolyzes a peptide bond at position 7 of the zymogen to make the active enzyme. E.A. DENNIS 2010 © Lipases and Phospholipases Lipases are unique because their substrates are lipids, not small molecules. • At low concentrations, DiC7PC forms monomers • At higher concentrations, it forms micelles. • The concentration at which micelles form is called the critical micelle concentration (CMC). DiC7PC PLA2 on DiC7PC Mixed Micelles with detergent Micelles V Monomers Zymogen Figure: Voet, D, Voet JG, Pratt CW (2006), Fundamentals of Biochemistry: Lif e at the Molecular Level, 2nd ed. Reprinted with permission of John Wiley & Sons, Inc. [S] CMC E.A. DENNIS 2010 © Lineweaver-Burk Plot of Lipase Activity • When TAG is mixed with water, it forms two layers. • When the water and TAG are shaken, they mix together forming microemulsions. • The finer the emulsion, the greater the activity of the enzyme due to increased surface area • 1/v versus surface area is the same for both Lipase Action Coarse emulsion Fine emulsion 1/v 1/[S] Fine Coarse 1/v 1/[Surface Area] E.A. DENNIS 2010 © Acknowledgement This tutorial is based on an evolving subset of lectures and accompanying slides presented to medical students in the Cell Biology and Biochemistry course at the School of Medicine of the University of California, San Diego. I wish to thank Dr. Bridget Quinn and Dr. Keith Cross for aid in developing many of the original slides, Dr. Eoin Fahy for advice in applying the LIPID MAPS nomenclature and structural drawing conventions [Fahy et al (2005) J Lipid Res, 46, 839-61; Fahy et al (2009) J Lipid Res, 50, S9-14] and Masada Disenhouse for help in adopting to the tutorial format. Edward A. Dennis September, 2010 La Jolla, California E.A. DENNIS 2010 ©