* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Chapter 5 Microbial Nutrition and Culture
Survey
Document related concepts
Metalloprotein wikipedia , lookup
Basal metabolic rate wikipedia , lookup
NADH:ubiquinone oxidoreductase (H+-translocating) wikipedia , lookup
Adenosine triphosphate wikipedia , lookup
Evolution of metal ions in biological systems wikipedia , lookup
Photosynthesis wikipedia , lookup
Citric acid cycle wikipedia , lookup
Biochemistry wikipedia , lookup
Electron transport chain wikipedia , lookup
Microbial metabolism wikipedia , lookup
Light-dependent reactions wikipedia , lookup
Transcript
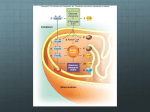
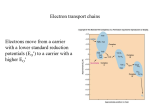
Chapter 5 Microbial Nutrition and Culture Siti Sarah Jumali (ext 2123) Room 3/14 [email protected] Groups of bugs based on energy capture and carbon source • AUTOTROPHY: Use carbon dioxide to synthesize organic molecules • Two types: 1. Photoautotrophs: obtain energy from light 2. Chemoautotrophs: obtain energy from oxidizing simple inorganic substance Groups of bugs based on energy capture and carbon source cont’d • HETEROTROPHY: Get carbon dioxide from ready made organic molecules • Two types: 1. Photoheterotrophs: obtain chemical energy from light 2. Chemoheterotrophs: obtain energy from breaking down ready-made organic compounds The main types of energy capturing Metabolism All microorganisms Inorganic CO2 = carbon source AUTOTROPH (Self feeders) Making own food by reducing CO2 Photoautotrophs Chemoautotrophs Organic compound = carbon source HETEROTROPH Using ready-made organic molecules for food Photoheterotrophs Chemoheterotrophs Examples of Energy Source Type Energy Source Carbon Source Examples Photolithotrophs Light CO2 Algae,Purple sulphur bacteri,green sulphur bacteria Photoorganotrophs Light Organic Compounds Purple non sulphur bacteria Chemolithotrophs Oxidation of inorganic compounds Chemoorganotrophs Oxidation of organic compounds Nitrifying bacteria,iron bacteria,H2 bacteria Organic Compounds Most bacteria, fungi,protozoa Photosynthesis and Respiration Metabolism • The sum of all chemical processes carried out by living organisms • Anabolism: rxn that requires energy in order to synthesize complex molecules from the simpler ones - (use energy and building blocks to build large molecules) • Catabolism: rxn that releases energy by breaking complex molecules into simpler ones which can be reused as building blocks - (provides energy and building blocks for anabolism) Metabolism: The sum of catabolism and anabolism Catabolism Larger molecules Smaller Molecules Anabolism Energy Metabolic Pathway • Glycolisis, fermentation, aerobic respiration and photosynthesis each consists of a series of chemical reaction • The product of one reaction serves as the substrate for the next: ABCD • Such chain of reactions is called a metabolic pathway: - Anabolic pathways make the complex molecules that form structure of cells, enzymes and molecules that control cells - Catabolic pathways capture energy in a form a cell can use Oxidation-Reduction Reactions • All catabolic reactions involve electron transfer which is directly related to oxidation and reduction (redox potential) • Redox reaction: An oxidation reaction paired with a reduction reaction - Oxidation: the loss of removal of electrons -Reduction: the gain of electrons Oxidation-Reduction Reactions Representative Biological Oxidations In biological systems, the electrons are often associated with hydrogen atoms. Biological oxidations are often dehydrogenation. Acronyms for oxidation and reduction: •Oxidation Is Losing Electrons, Reduction Is Gaining Electrons: OIL RIG •Losing Electrons Oxidation, Gaining Electrons Reduction: LEO the lion. GER! or LEO says GER •Electron Loss Means Oxidation: ELMO Metabolic Pathways of Energy Production 13 Metabolic Pathways of Energy Production (b) Energy Transfer by Carrier Molecules • Carrier molecules such as Cytochrome (cyt) and some coenzymes carry energy in the form of electrons in many biochemical reactions • Coenzymes such as FAD carry whole hydrogen atoms (electrons together with protons); NAD carries one hydrogen atom and one “naked” electron • When co-enzymes are reduced, they increase in energy, when they are oxidized, they decrease in energy. Energy Generation of ATP • ATP is generated by the phosphorylation of ADP Energy ADP + Pi + Energy ATP • In cells, energy is provided by the hydrolysis of ATP ATP ADP + Pi + Energy Energy Generation of ATP 1. Substrate level Phosphorylation: Energy from the transfer of a high energy PO4 to ADP generates ATP C-C-C-P + ADP C-C-C + ATP 2. Oxidative Phosphorylation: Energy relseased from transfer of electrons (oxidation) of one compound to another (reduction) is used to generate Atp in the electron transport chain 3. Photophosporylation: Light causes chlorophyll to give up electrons. Energy released from transfer of electrons (oxidation) of chlorophyll trough a system of carrier molecules is used to generate ATP Overview of Respiration vs Fermentation Carbohydrate Catabolism • The breakdown of carbohydrate to release energy involves 1. Glycolisis (cytoplasm) 2. Krebs cycle (mitochondrion) 3. Electron transport chain Glycolysis • Glycolysis (Embden Meyerhof pathway) is the metabolic pathway used by most autotrophic and heterotrophic organismsm to begin breakdown of glucose • Does not require oxygen, but occur in precense or absence of oxygen • Overall chemical reaction of Glycolysis Glycolysis: Oxidation of Glucose Glucose 2ATP 2 NAD+ 2ADP 2NADH + 2H+ two Glyceraldehyde-3-PO4 4 ADP 4 ATP two Pyruvate 23 P O CH2 HO CH2 O OH CH2 O P O PO4 OH HO OH OH OH glucose OH fructose-1,6-diphosphate CHO 2 H C OH CH2O P 2 glyceraldehyde-3-phosphate 24 2 ADP + 2 Pi 2 ATP CHO CHO 2 H C OH CH2O P 2 H C O CH3 2 glyceraldehyde-3-phosphate 2 pyruvate 2 NAD+ 2 NADH + 2 H+ 25 Glycolysis: Oxidation of Glucose Glycolysis generates 2 ATP molecules and 2 NADH + 2 H+ Two ATP used in adding phosphate groups to glucose and fructose-6-phosphate (- 2 ATP) Four ATP generated in direct transfer to ADP by two 3-C molecules (+ 4 ATP) Glucose + 2 ADP + 2 Pi + 2 NAD+ 2pyruvate + 2 ATP + 2 NADH + 2 H+ 26 Pathways for Pyruvate Aerobic conditions O || CH3–C –COO- + NAD+ + CoA pyruvate O || CH3–C –CoA + CO2 + NADH + H+ acetyl CoA 27 Pathways for Pyruvate Anaerobic conditions (No O2 available) Reduce to lactate to replenish NAD+ for glycolysis O || CH3–C –COO- + NADH + H+ OH | CH3–CH –COO- + NAD+ pyruvate lactate enzyme: lactate dehydrogenase 28 Glycolysis Alternative to Glycolysis • Pentose phosphate pathway a. Uses pentoses and NADPH b. Operates with glycolisis • Entner-Doudoroff pathway a. Produces NADPH and ATP b. Does not involve glycolisis c. Pseudomonas, Rhizobium, Agrobacterium Intermediate step • Pyruvic acid (from glycolysis) is oxidized and decarboxylated The Krebs Cycle/ The Citric acid cycle (TCA cycle) • Oxidation of acetyl Co-A produces NADH and FADH2 (mitochondrion) The Electron Transport Chain • An electron transport chain (ETC) couples electron transfer between an electron donor (such as NADH) and an electron acceptor (such as O2) to the transfer of H+ ions (protons) across a membrane. • A series of oxidation-reduction reactions, the electron transport chain (ETC) performs 2 basic functions: 1. Accepting electrons from an electron donor and transferring them to an electron acceptor 2. Conserving for ATP synthesis some of the energy released during the electron transfer • A series of carrier molecules that are, in turn oxidized and reduced as electrons are passed down the chain • Energy released can be used to produce ATP by chemiosmosis The Electron Transport Chain The Electron Transport Chain Chemiosmosis • Electrons from the hydrogen atoms removed from the reactions of the Krebs cycle are transferred through the electron transport system • Electron transport creates the H potential across the membrane • Combination of hydrogen/electron carriers Chemiosmosis Chemiosmosis Questions?