* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Figure 11-1
Oxidative phosphorylation wikipedia , lookup
Gene regulatory network wikipedia , lookup
Microbial metabolism wikipedia , lookup
Biochemical cascade wikipedia , lookup
Metabolic network modelling wikipedia , lookup
Metalloprotein wikipedia , lookup
Evolution of metal ions in biological systems wikipedia , lookup
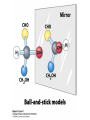
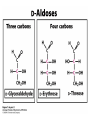
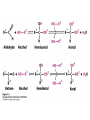
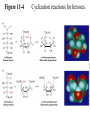
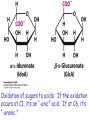
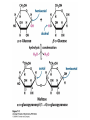
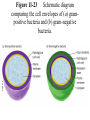
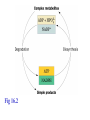
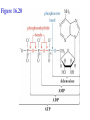
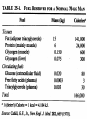
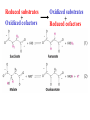
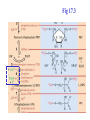
Winter 2011 Biol/Chem 472 Metabolism • • • • • Instructor: Gerry Prody Office CB444 Office hrs: TBA [email protected] http://lightning.chem.wwu.edu/dept/facstaff/prody/ prody-472.htm Welcome to Biochemistry The required textbook for this course is: LEHNINGER Principles of Biochemistry 5/e by David L. Nelson and Michael M. Cox (©2008, W.H. Freeman & Company) Free Companion Website This site is chock full of resources to help you succeed in the course. www.whfreeman.com/lehninger5e Student Media Resources www.whfreeman.com/lehninger5e • Interactive quizzes help you practice for exams • Animated Enzyme Mechanisms and Animated Biochemical Techniques help you understand key mechanisms and techniques at your own pace • Molecular Structure Tutorials allow you to explore in more depth the molecular structures included in the text • Living Graphs illustrate key equations from the book allowing you to do what if scenarios by changing the parameters • Lecture Companion Art allow you to print figures and tables for note-taking and review Additional Saleable Supplement: The Absolute Study Guide & Solutions Manual It combines an innovative study guide with a reliable solutions manual providing extended solutions to the end-of-chapter problems in the textbook. It includes for each chapter: - Major Concepts: A roadmap through the chapter - What to Review: Questions that recap key points from previous chapters - Discussion Questions: Designed for individual review, study groups, or classroom discussion - A Self-Test: “Do you know the terms?”; crossword puzzles; multiple-choice, fact-driven questions; and questions that ask students to apply their knowledge To learn more contact your local bookstore. Need support? 1-800-936-6899 Monday-Friday, 9-5 EST [email protected] What is Biochemistry? • the systematic torture of students with copious incomprehensible jargon, cryptic fomulae, and impossible insoluble problems. “Biochemistry is the study of Life as a process that can be understood.” Primary Objective: understand the molecular mechanisms that constitute the living state (“Molecular Logic”) Lehninger: “Molecular Logic” “A living cell is a self-assembling, self-regulating, self-replicating isothermal open system of organic molecules operating on a principle of maximum economy of parts and processes; it promotes many consecutive, linked organic reactions for the transfer of energy and for the synthesis of its own components by means of organic catalysts that it produces itself.” Biol/Chem 471; Biol/Chem 472 ; Biol/Chem 473 Elemental composition of the earth’s surface, including crust, oceans and atmosphere. Element Oxygen Silicon Aluminum Iron Calcium Sodium Potassium Magnesium Hydrogen Titanium Chlorine Carbon All others Percent by mass 49.1 26.1 7.5 4.7 3.4 2.6 2.4 1.9 0.88 0.58 0.19 0.09 0.56 Page 29 Table 1-3 Elemental Composition of the Human Body. Map of the major metabolic pathways in a typical cell. Biol/Chem 472 Expected Outcomes • draw enzymatic reactions correctly • correctly calculate DGº’ and DG for a given step or a series of steps in a pathway • rationalize and/or predict features of pathway regulation and describe regulatory mechanisms • recognize how concentrations of metabolites are regulated and the impact that changes in flux and/or concentration will have on other processes. Note that figures labeled as Ch 11 come from Voet and Voet. You can find analogous figures in your text. Page 357 Figure 11-1 The stereochemical relationships, shown in Fischer projection, among the D-aldoses with three to six carbon atoms. Page 358 Figure 11-2 The stereochemical relationships among the D-ketoses with three to six carbon atoms. Page 358 Figure 11-3 The reactions of alcohols with (a) aldehydes to form hemiacetals and (b) ketones to form hemiketals. Figure 11-4 Cyclization reactions for hexoses. Page 359 Figure 11-5 The anomeric monosaccharides a-D-glucopyranose and b-D-glucopyranose, drawn as both Haworth projections and ball-andstick models. Oxidation of sugars to acids: If the oxidation occurs at C1, it’s an “-onic” acid. If at C6, it’s “-uronic.” Page 361 Figure 11-8 The acid-catalyzed condensation of a-D-glucose with methanol to form an anomeric pair of methyl-Dglucosides. Some hexose derivatives important in biology Figure 11-11 N-Acetylneuraminic acid in its linear and pyranose forms. Page 363 Sialic acid BOX 7-1 FIGURE 1 The glucose oxidase reaction, used in the measurement of blood glucose. A second enzyme, a peroxidase catalyzes the reaction of the H2O2 with a colorless compound to produce a colored product, which is measured spectrophotometrically. What is the second enzyme? 474 folks… BOX 7-1 FIGURE 2 The nonenzymatic reaction of glucose with a primary amino group in hemoglobin begins with (1) formation of a Schiff base, which (2) undergoes the Amadori rearrangement to generate a stable product; (3) this ketoamine can further cyclize to yield GHB. (4) Subsequent reactions generate advanced glycation end products (AGEs), such as ε-N-carboxymethyllysine and methylglyoxal, compounds that (5) can damage other proteins by cross-linking them, causing pathological changes. Which of these are reducing sugars? Which are nonreducing? Page 365 Figure 11-13 Electron micrograph of the cellulose fibers in the cell wall of the alga Chaetomorpha melagonium. Page 365 Figure 11-14 The primary structure of cellulose. Page 365 Figure 11-15 Proposed structural model of cellulose. Cell wall architecture Pectins extensin Common sugars found in plant polysaccharides Pectin structures Cross-bridging and esterification in pectins A spotted June beetle (Pelidnota punctata), showing its surface armor (exoskeleton) of chitin. Page 366 Figure 11-16 Structure of chitin. Page 366 Figure 11-17a a-Amylose. The D-glucose residues of a-amylose are linked by a(1 4) bonds (red). Page 366 Figure 11-17b a-Amylose. This regularly repeating polymer forms a lefthanded helix. Page 367 Figure 11-18a Amylopectin. Its primary structure near one of its a(1 6) branch points (red). Page 367 Figure 11-18b Amylopectin. (b) Its bushlike structure with glucose residues at branch points indicated in red. Page 368 Figure 11-20 The disaccharide repeating units of the common glycosaminoglycans. Page 373 Figure 11-23 Schematic diagram comparing the cell envelopes of (a) grampositive bacteria and (b) gram-negative bacteria. Both + and - walls NAM Page 373 NAG Figure 11-24a Chemical structure of peptidoglycan. (a) The repeating unit of peptidoglycan. Page 373 Figure 11-24b Chemical structure of peptidoglycan. (b) The S. aureus bacterial cell wall peptidoglycan. Figure 11-25 Structure of penicillin. From yeast Page 374 Prevents crosslinking of peptides Alexander Fleming Page 374 Figure 11-26 Enzymatic inactivation of penicillin. Page 376 Figure 11-29a N-Linked oligosaccharides. (a) All N-glycosidic protein attachments occur through a b-N-acetylglucosamino–Asn bond to Asn–X–Ser/Thr. Page 376 Figure 11-29c N-Linked oligosaccharides. (c) Some examples of Nlinked oligosaccharides. Page 376 Figure 11-30 Some common O-glycosidic attachments of oligosaccharides to glycoproteins (red). Figure 11-33a The surfaces of (a) a normal mouse cell as seen in the electron microscope. (b) a cancerous cell as seen in the electron microscope. Page 378 Agglutinated with Conconavalin A--specific for glc and man a b Living cells are not at equilibrium! Concentrations of reactants and products are typically far from the equilibrium values (Q Keq). DG = DG + RTlnQ o' We must consider “steady state” concentrations of these species for the determination of DG. Homeostatic conditions Fig 16.2 Catabolic pathways Anabolic pathways See Figure 16.3 Figure 16.20 Figure 16.25 ° °’ Table 16.3 31P NMR of human muscle: Before exercise 1 min of exercise Pi ATP phosphocreatine 19 min of exercise gab 10 min after exercise Reduced substrates + Oxidized cofactors Oxidized substrates + Reduced cofactors Fig 17.3 Least oxidized Most oxidized Page 567 Figure 16-21b Some overall coupled reactions involving ATP. (b) The phosphorylation of ADP by phosphoenolpyruvate to form ATP and pyruvate. Biol/Chem 472 Expected Outcomes • draw enzymatic reactions correctly • correctly calculate DGº’ and DG for a given step or a series of steps in a pathway • rationalize and/or predict features of pathway regulation and describe regulatory mechanisms • recognize how concentrations of metabolites are regulated and the impact that changes in flux and/or concentration will have on other processes. “Alfonse, Biochemistry makes my head hurt!!” \