* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download anticoagulantpresent..
Pharmacokinetics wikipedia , lookup
Pharmaceutical industry wikipedia , lookup
Discovery and development of proton pump inhibitors wikipedia , lookup
Pharmacognosy wikipedia , lookup
Metalloprotease inhibitor wikipedia , lookup
Prescription costs wikipedia , lookup
Discovery and development of integrase inhibitors wikipedia , lookup
Drug-eluting stent wikipedia , lookup
Psychedelic therapy wikipedia , lookup
Drug interaction wikipedia , lookup
Psychopharmacology wikipedia , lookup
Neuropharmacology wikipedia , lookup
Pharmacogenomics wikipedia , lookup
Neuropsychopharmacology wikipedia , lookup
Discovery and development of direct Xa inhibitors wikipedia , lookup
Discovery and development of direct thrombin inhibitors wikipedia , lookup
Blood Drugs
• describes drugs that are useful in treating
three important dysfunctions of blood:
thrombosis, bleeding, and anemia.
Drugs used in treatment of
thrombosis
Objectives
• To learn how Blood Clots are formed
and broken down ?
• What drugs can be used to regulate
clotting ?
THROMBOSIS
• Thrombosis is the formation of an
unwanted clot within a blood vessel.
• it is the most common abnormality of
hemostasis.
Consequences of thrombus
consequences
angina
Myocardial
infarction
stroke
Deep venous
thrombosis
Thrombus versus embolus
• A clot that adheres to a vessel wall is called
a thrombus
• whereas an intravascular clot that floats in
the blood is termed an embolus.
• Thus, a detached thrombus becomes an
embolus.
• Both thrombi and emboli are dangerous,
because they may occlude blood vessels
and deprive tissues of oxygen and nutrients.
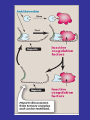
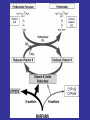
Blood Clotting
• Vascular Phase
• Platelet Phase
• Coagulation Phase
• Fibrinolytic Phase
Vascular Phase
Vasoconstriction
Exposure of tissues activate Tissue
factor and initiate coagulation
Tissue Factor
Platelet activation
1- resting platelets:
• In the absence of injury, resting platelets
circulate freely, because the balance of
chemical signals indicates that the
vascular system is not damaged.
AChemical
mediators
synthesized
by
endothelial cells:
• Chemical mediators, such as prostacyclin and
nitric oxide, are synthesized by intact endothelial
cells and act as inhibitors of platelet aggregation.
• Prostacyclin (prostaglandin I2) acts by binding to
platelet membrane receptors that are coupled to
the synthesis of cyclic adenosine monophosphate
(cAMP).
• Elevated levels of intracellular cAMP are
associated with a decrease in intracellular Ca2+.
This leads to inhibition of platelet activation and
inhibit release of platelet aggregation agents.
• Damaged endothelial cells synthesize less
prostacyclin, resulting in a localized
reduction in prostacyclin levels.
• The binding of prostacyclin to platelet
receptors is decreased, resulting in lower
levels of intracellular cAMP, which leads to
platelet aggregation.
B- Roles of thrombin, thromboxane, and collagen:
• The platelet membrane also contains receptors that
can bind thrombin, thromboxane2, and exposed
collagen, that when occupied triggers platelet
aggregation.
• In the intact, normal vessel, circulating levels of
thrombin and thromboxane are low
• the intact endothelium covers the collagen in the
subendothelial layers.
• The corresponding platelet receptors are thus
unoccupied and remain inactive; as a result,
platelet activation and aggregation are not initiated.
2- Platelet adhesion
• When the endothelium is injured, platelets
adhere to and virtually cover the exposed
collagen of the subendothelium.
• This triggers a complex series of chemical
reactions, resulting in platelet activation
• 3- platelet activation
• Receptors on the surface of the adhering platelets
are activated by the collagen of the underlying
connective tissue.
• This causes morphologic changes in the platelets
and the release of platelet granules containing
chemical mediators, such as:
• adenosine diphosphate (ADP)
• thromboxane A2
• Serotonin
• platelet-activation factor
• thrombin.
• .
• These signaling molecules bind to receptors in the
outer membrane of resting platelets circulating
nearby.
• The previously dormant platelets become
activated and start to aggregate
4- Platelet aggregation
Platelet activation leads to :
1) activation of the glycoprotein (GP) IIb/IIIa
receptors that bind fibrinogen and, ultimately,
regulate platelet-platelet interaction and thrombus
formation.
Fibrinogen, simultaneously binds to GP IIb/IIIa
receptors on two separate platelets, resulting in
platelet cross-linking and platelet aggregation.
This leads to an avalanche of platelet aggregation,
because each activated platelet can recruit other
platelets
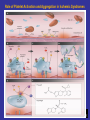
Role of Platelet Activation and Aggregation in Ischemic Syndromes
Bhatt D. N Engl J Med 2007;357:2078-2081
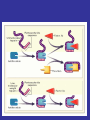
ANTIPLATELET THERAPY
Platelet aggregation inhibitors
• Platelet aggregation inhibitors decrease
the formation or the action of chemical
signals that promote platelet aggregation.
• The most important of these is the GP
IIb/IIIa receptor that ultimately regulates
platelet-platelet interaction and thrombus
formation.
• The
platelet
aggregation
inhibitors
described below act by:
1.Inhibit cyclooxygenase-1 (COX-1) or
2.block GP IIb/IIIa or
3.block ADP receptors
Thereby interfering in the signals that
promote platelet aggregation.
Since these agents have different
mechanisms of actions, synergistic or
additive effects may be achieved when
agents from different classes are
combined.
These agents are beneficial in
1. the prevention and treatment of occlusive
cardiovascular diseases
2. in the maintenance of vascular grafts and
arterial patency
3.As adjuncts to thrombin inhibitors or
thrombolytic therapy in myocardial infarction.
A- aspirin
• Activation of platelets results in stimulation of platelet
membrane phospholipases that liberate arachidonic
acid from membrane phospholipids.
• Arachidonic acid is converted into thromboxane A2
by COX enzyme
• Activated platelets releases thromboxane which
activates further platelets.
• Aspirin inhibits thromboxane A2 synthesis by
irreversible inhibition of platelet COX enzyme.
• This shifts the balance of chemical mediators to favor
the antiaggregatory effects of prostacyclin, thus
impeding platelet aggregation.
• \
• The aspirin-induced suppression of thromboxane
last for the life of the anucleated platelet;
approximately 7 to 10 days.
• Aspirin is currently employed in the prophylactic
treatment of :
1. transient cerebral ischemia
2. to reduce the incidence of recurrent myocardial
infarction
3. and to decrease mortality in pre and post
myocardial infarct patients.
• The recommended dose of aspirin ranges from 81
to 325 mg, with side effects determining the dose
chosen.
• Side effects:
• Bleeding time is prolonged by aspirin treatment,
causing complications that include increased
incidence of hemorrhagic stroke as well as
gastrointestinal bleeding, especially at higher doses
of the drug.
•
• NSAID, such as ibuprofen, inhibit COX-1 by
transiently competing at the catalytic site. Ibuprofen, if
taken concomitantly with, or 2 hours prior to aspirin,
can obstruct the access of aspirin to the catalytic site
and, thereby, antagonize the platelet inhibition by
aspirin.
• Therefore, aspirin should be taken at least 30
minutes before ibuprofen or at least 8 hours after
ibuprofen.
• Although celecoxib (a selective COX-2 inhibitor)
does not interfere in the antiaggregation activity of
aspirin there is some evidence that it may
contribute to cardiovascular events by shifting the
balance of chemical mediators in favor of
thromboxane A2.
• Aspirin: Primary prevention of MI in high
risk persons
Secondary prevention of MI, & stroke
• Clopidogrel: for persons who can’t take
aspirin
• Aspirin+clopidogrel:
Acute
coronary
syndromes
• Treatment failures occur with aspirin therapy;
approximatly 40% of human patients on aspirin
therapy develop an ischemic event.
• There are many possible causes for this. Aspirin
is a relatively weak inhibitor of platelet function.
It inhibits only one pathway of platelet activation
and aggregation.
• Moreover, platelet aggregation is only one
pathway of thrombus formation.
• True „aspirin resistance‟ refers to failure of
aspirin to inhibit TXA2 production.
Potential mechanisms include:
(1) decreased bioavailability
(2) competition with other NSAIDs
(3) accelerated platelet turnover, introducing
newly-formed, non aspirinated platelets into
the circulation
(4) TXA2 production by the aspirininsensitive
COX-2
in
newly-formed
platelets.
Ticlopidine and clopidogrel
Mechanism of action:
• These drugs irreversibly inhibit the binding
of ADP to its receptors on platelets
Therapeutic use:
• Ticlopidine is approved for the prevention of
transient ischemic attacks and strokes for
patients with prior cerebral thrombotic event.
It is also used as adjunct therapy with
aspirin following coronary stent implantation
to decrease the incidence of stent
thrombosis.
• However, due to its life-threatening
hematologic adverse reactions, including
neutropenia/agranulocytosis,
thrombotic
thrombocytopenic purpura (TTP), and
aplastic anemia, it is generally reserved for
patients who are intolerant to other
therapies
• Clopidogrel is approved for prevention of
atherosclerotic events following recent
myocardial infarction, stroke, or established
peripheral arterial disease. It is also
approved for prophylaxis of thrombotic
events in acute coronary syndrome
(unstable angina).
• Additionally, clopidogrel is used to prevent
thrombotic
events
associated
with
percutaneous coronary intervention with or
without coronary stent.
• Compared to ticlopidine, clopidogrel is the
preferred agent in ischemic heart disease
events, because there is more data to
support use of clopidogrel in these cardiac
patients.
• Furthermore, clopidogrel has a better
overall side-effect profile although TTP
may also occur with this agent.
Pharmacokinetics:
• Food interferes with the absorption of
ticlopidine but not with clopidogrel.
• After oral ingestion, both drugs are
extensively bound to plasma proteins.
• They undergo hepatic metabolism by the
cytochrome P450 system to active
metabolites that are yet to be identified.
• The maximum effect is achieved in 3 to 5
days
• when treatment is suspended, the platelet
system requires time to recover.
Side effects
• Ticlopidine has a black box warning due to
the severe hematologic adverse reactions
associated with its use.
• Both drugs can cause prolonged bleeding
for which there is no antidote.
• Serious adverse effects of ticlopidine
include neutropenia, TTP, and aplastic
anemia
requiring
frequent
blood
monitoring, especially during the first 3
months of treatment.
Drug interaction:
• Because these drugs can inhibit
cytochrome P450, they may interfere with
the metabolism of drugs such as
phenytoin,
tolbutamide,
warfarin,
fluvastatin, and tamoxifen if taken
concomitantly.
• Indeed, phenytoin toxicity has been
reported when taken with ticlopidine.
Abciximab
• chimeric monoclonal antibody
• composed of the constant regions of
human immunoglobulin joined to the Fab
fragments of a murine monoclonal
antibody directed against the GP IIb/IIIa
complex.
• By binding to GP IIb/IIIa, the antibody
blocks the binding
of
fibrinogen;
consequently, aggregation does not occur
• Abciximab is given intravenously along with heparin
or aspirin as an adjunct to percutaneous coronary
intervention for the prevention of cardiac ischemic
complications.
• After cessation of infusion, platelet function gradually
returns to normal, with the antiplatelet effect
persisting for 24 to 48 hours.
• The major adverse effect of abciximab therapy is the
potential for bleeding, especially if the drug is used
with anticoagulants or if the patient has a clinical
hemorrhagic condition.
• Abciximab is expensive, limiting its use in some
settings.
Eptifibatide and tirofiban
• These two antiplatelet drugs act similarly
to abciximab, blocking the GP IIb/IIIa
receptor.
• These compounds, like abciximab, can
decrease the incidence of thrombotic
complications associated with acute
coronary syndromes.
• When intravenous infusion is stopped,
these agents are rapidly cleared from the
plasma, but their effect can persist for as
long as 4 hours.
• Only
intravenous
formulations
are
available, because oral preparations of GP
IIb/IIIa blockers are too toxic.
• Eptifibatide and its metabolites are
excreted by the kidney.
• Tirofiban is excreted unchanged by the
kidney.
• The major adverse effect of both drugs is
bleeding.
Dipyridamole
• Dipyridamole
[dye-peer-ID-a-mole],
a
coronary
vasodilator,
is
employed
prophylactically to treat angina pectoris.
• It is usually given in combination with
aspirin or warfarin
• it is ineffective when used alone.
• Dipyridamole increases intracellular levels
of cAMP by inhibiting cyclic nucleotide
phosphodiesterase.
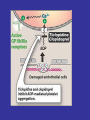
Sites of Action of Antiplatelet Therapy on Mechanisms of Platelet Activation and Aggregation
Schulman, S. P. JAMA 2004;292:1875-1882.
Copyright restrictions may apply.
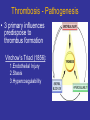
Thrombosis - Pathogenesis
• 3 primary influences
predispose to
thrombus formation
Virchow’s Triad (1856):
1.Endothelial Injury
2.Stasis
3.Hypercoagulability
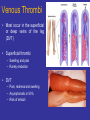
Venous Thrombi
• Most occur in the superficial
or deep veins of the leg
(DVT)
• Superficial thrombi
– Swelling and pain
– Rarely embolize
• DVT
– Pain, redness and swelling
– Asymptomatic in 50%
– Risk of emboli
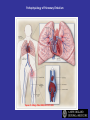
Pathophysiology of Pulmonary Embolism
Tapson V. N Engl J Med 2008;358:1037-1052
anticoagulants
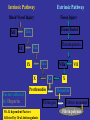
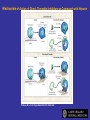
Coagulation Phase
Two major pathways
Intrinsic pathway
Extrinsic pathway
Both converge at a common point
• Biosynthesis of these factors are dependent on Vitamin K1
and K2
Normally inactive and sequentially activated
Intrinsic Pathway
All clotting factors
Extrinsic Pathway
Initiating factor is
are within the blood
outside the blood
vessels
vessels - tissue
Clotting slower
Activated partial
thromboplastin test
(aPTT)
factor
Clotting - faster - in
Seconds
Prothrombin test (PT)
Intrinsic Pathway
Extrinsic Pathway
Tissue Injury
Blood Vessel Injury
Tissue Factor
XIIa
XII
Thromboplastin
XIa
XI
IXa
IX
Xa
X
Factors affected
By Heparin
VIIa
Prothrombin
Vit. K dependent Factors
Affected by Oral Anticoagulants
Fibrinogen
VII
X
Thrombin
Fribrin monomer
Fibrin polymer
The Coagulation and Fibrinolytic Pathways
Present on platelets’
surfaces. Act by
accelerating
thrombus formation.
Activated factor X (FXa) + FVa + Ca++ + phospholipids
Prothrombin thrombin
Fibrinogen fibrin blood clot
This complex and Ca2+ comprise
the prothrombinase complex
Thrombin stimulates platelet aggregation
Phospholipids on platelets stimulate clot formation
Platelets aggregation ↔ coagulation
• The coagulation process that generates
thrombin consists of two interrelated
pathways the extrinsic and the intrinsic
systems.
• The extrinsic system, which is probably the
more important system in vivo, is initiated by
the activation of clotting Factor VII by tissue
factor, or thromboplastin.
• Tissue factor is a lipoprotein that is expressed
by cells at the site of vascular injury.
• The intrinsic system is triggered by the activation of
clotting Factors its contact in vitro with glass or
highly charged surfaces.
• Intrinsic and extrinsic activation of the coagulation
cascade leads to the generation of thrombin, the
activation of fibrinogen, the release of fibrinopeptides,
the formation of soluble fibrin, and finally, the
formation of cross-linked, insoluble fibrin.
• (The activated form of the factor is indicated by "a.")
• It is important that coagulation is restricted
to the local site of vascular injury.
• Endogenously, there are several inhibitors
of coagulation factors, including protein C,
protein S, antithrombin III, and tissue
factor pathway inhibitor.
• The mechanism of action of several
anticoagulant agents, including heparin
and heparin-related products, involves
activation of these endogenous inhibitors
(primarily antithrombin III).
anticoagulants
• The anticoagulant drugs either:
1. inhibit the action of the coagulation factors
(the thrombin inhibitors, such as heparin
and heparin-related agents)
2. interfere with the synthesis of the
coagulation factors (the vitamin K
antagonists, such as warfarin).
http://www.hopkinsmedicine.org
/hematology/coagulation.swf
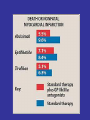
Hemostasis requires a fine balance between
procoagulant and regulatory factors
Deficiency
Coagulation
Proteins/
Platelets/
Vessel wall
PC
PS
ATIII…
Thrombosis
Deficiency/
Abnormality
Bleeding
Anticoagulants FACTORY
Scheme of anticoagulant drugs
Anticoagulant
drugs
oral
warfarin
injected
Direct acting:
Lepirudin
Antithrombin
III dependent:
heparin
Low molecular
weight heparin
•
Injected; THROMBIN
INHIBITORS
Thrombin inhibitors
can either inactivate
thrombin directly or block thrombin formation
• Thrombin can be inhibited irreversibly by
glycosaminoglycans like heparin through an
antithrombin III-dependent mechanism
• The enzyme can be inhibited reversibly by
hirudin and hirudin derivatives in an antithrombin
III-independent manner (direct acting)
• In
addition
to
inhibiting
thrombin,
glycosaminoglycans
also
block
thrombin
generation
Antithrombin-III Dependent
Thrombin Inhibitors
Standard Unfractionated Heparin (UFH)
Heparin is a mixture of glycosaminoglycan molecules,
which are heterogenous in molecular size
The mean molecular weight of heparin is 15,000 D
Antithrombin III (ATIII) binding is necessary for its
anticoagulant activity
Antithrombin III (ATIII) is a slow endogenous
progressive inhibitor of thrombin and other clotting
enzymes (Xa)
Mode of Action of Heparin
It binds to ATIII through a unique
pentasaccharide conformational change
in ATIII ↑ activity of ATIII
N.B. ATIII alone can inhibit thrombin but
in a very slow reaction
Heparin acts as a template to create
(thrombin-ATIII complex)
N.B. only 1 ATIII bind to 1 thrombin
(1:1)
Then heparin dissociates and is reused again
Heparin inactivates thrombin by binding
both ATIII and thrombin
To inactivate thrombin
1. Heparin binds to ATIII by the unique penta-saccharide
2. Also binds to thrombin through the heparin-binding domain
• heparn also inactivate factor Xa, but in such case it binds
only with ATIII through its pentasaccharide sequence
Every heparin molecule
contains :
Pentasaccharide +
heparin binding domain
Anti-IIa activity = Anti-Xa activity
Low Molecular Weight Heparins
(LMWHs)
Low molecular weight heparin have a mean
molecular weight of 5000 D.
Prepared by controlled chemical or
enzymatic depolymerization of standard
unfractioned heparin are about ⅓ the size
of starting material
Enoxaparin is the most used LMWH
Mechanism of Action of Low Molecular
Weight Heparin (LMWH)
They contain pentasaccharide inactivation of Factor Xa
In contrast, only 25% to 50% of LMWH molecules that have
the pentasaccharide sequence are long enough to interact
with both ATIII & thrombin
25% of LMWH can interact with
both ATIII and thrombin
The rest only inactivate factor X
Anti-IIa < Anti-Xa activity
Pharmacokinetic Profile of LMWH
Bioavilability Bbioavailability (90% vs. 20% of
heparin)
LMWH exhibit less binding to plasma proteins &
cell surfaces (better than heparin)
more predictable anticoagulant response
Laboratory monitoring of LMWH activity is not
required
LMWH has low resistance in comparison to
heparin
T1/2 = 4 hours (more than heparin)
Given at fixed doses once to twice daily by S.C.
route, and is given for both inpatients as well as
for outpatients.
Comparison of UFH & LMWH
Character
UFH
Average Mol wt
15,000
Anti-Xa/anti-IIa activity
1/1
aPTT monitoring required
Yes
Inactivation of platelet-bound Xa
No
Protein binding
Powerful )4+)
LMWH
5,000
2-4/1
No
Yes
Weak (+)
Endothelial cell binding
Dose-dependent clearance
Powerful )4+)
Yes
No
No
Elimination half-life
30-150 min
2-5 times
longer
Biophysical Limitations of
Heparin and LMWH
Both heparin and LMWH can’t degrade fibrinbound thrombin (only free thrombin is degraded)
nor Factor Xa within the prothrombinase complex.
Therapeutic Uses
o Heparin should be given either IV or S.C. injection.
o onset of action: few minutes (IV) 1-2 hours (S.C.)
o LMWHs are given by S.C. route
o I.M. injection hematoma formation (thus is avoided)
Treatment of deep vein thrombosis
Treatment of pulmonary embolism
Prevention of postoperative venous thrombosis in
patients with acute MI phase or one undergoing elective
surgery (not emergency surgery)
Reduction of coronary artery thrombosis after
thrombolytic treatment
Heparin is the anticoagulant of choice in pregnant women
• Heparin and LMWHs are the anticoagulants of
choice for treating pregnant women with prosthetic
heart valves or venous thromboembolism,
because these agents do not cross the placenta
(due to their large size and negative charge).
• Heparin has the advantage of speedy onset of
action, which is rapidly terminated on suspension
of therapy.
• However, it is being supplanted by the LMWHs,
such as enoxaparin and dalteparin, because they
can be conveniently injected subcutaneously on a
patient weight basis, have predictable therapeutic
effects,
and
have
a
more
predictable
pharmacokinetic profile
Adverse Effects
• Bleeding: they both lead to bleeding but the
bleeding is less in LMWH
To treat bleeding: inject antidote protamine
sulphate (1mg IV for each 100 units of UFH)
(reversal effect)
• Thrombosis
and
heparin
induced
Thrombocytopenia (HI)T: HIT is caused by the
formation of abnormal antibodies that activate
platelets. so someone receiving heparin develops
new or worsening thrombosis, or if the platelet
count falls.
HIT is a life threatening immune reaction
Occurs in 3% of patients
Usually occurs a week from starting heparin
therapy
LMWHs, though of lower risk, are contraindicated
with HIT.
For patients with HIT we
use lepirudin
How does HIT occur?
• Heparin injection
immune reaction with
body produce antibody against heparin
& also bind to platelet receptor
activation of platelet
thrombosis
Osteoporosis occurs with large doses of UFH
>20,000 U/day for 6 months or longer (chronic
use)
Hyperkalemia rarely occurs with UFH
It is attributed to inhibition of aldosterone
secretion
It is reversible by therapy discontinuation
Diabetic & renal failure patients are at higher risk
Hypersensitivity: (Antigenicity due to animal
source)
rarely occurring reactions include urticaria, rash,
rhinitis, angioedema & reversible alopecia(hair
loss)
HEPARIN TEST of CONTROL
ACTIVATED PARTIALTHROMBOPLASTIN
TIME
OR
aPTT or PTT
A THERAPEUTIC VALUE, ~ 0.3 u/ml
REVERSAL
UFH – Protamine
LMWH – Protamine not fully effective
Stead L and Judson K. N Engl J Med 2006;355:e7
Other Injectable Antithrombotic Agents
• Fondaparinux, a pentasaccharide, is an AT-IIIdependent selective for factor Xa
Prevents venous thrombosis associated with
orthopedic surgery
Administered > 6 hours postoperatively and the
dose is adjusted for patients with renal
impairment.
FONDAPARINUX
• It is well absorbed from the subcutaneous route
with a predictable pharmacokinetic profile.
• Fondaparinux requires less monitoring than
heparin.
• Fondaparinux is eliminated in urine mainly as
unchanged drug with an elimination half-life of 17
to 21 hours.
• It is contraindicated in patients with severe renal
impairment (<30 mL/min).
• Bleeding episodes are the major side effect of
fondaparinux therapy.
• Thrombocytopenia, is not a problem, and this
agent may be used in patients with HIT.
Clinically Approved Direct Thrombin
Inhibitors
•
•
•
•
•
Lepirudin, recombinant hirudin*-like peptide.
Direct acting thrombin inhibitor
Used in HIT patients (IV injection)
Has renal clearance
It acts on free thrombin and thrombin bound to fibrin
It has potential use in unstable angina patients and after
thrombolysis
* hirudin: is a leech derived
anticoagulant
It binds to active site and substrate
site of thrombin
• Bleeding is the major adverse effect of treatment
with lepirudin, and it can be exacerbated by
concomitant thrombolytic therapy, such as
treatment with streptokinase or alteplase.
• About half the patients receiving lepirudin develop
antibodies. However, the drug-antibody complex
retains anticoagulant activity.
• Because renal elimination of the complex is slower
than that of the free drug, the anticoagulant effect
may be increased. It is important to monitor the
aPTT and renal function when a patient is
receiving lepirudin.
Argatroban
• is metabolized in the liver and has a half life of
about 50 minutes.
• It is monitored by aPTT. The patient's hemoglobin
and hematocrit must also be monitored.
• Because argatroban is metabolized in the liver, it
may be used in patients with renal dysfunction but
it should be used cautiously in patients with
hepatic impairment.
• As with other agents in this class, the major side
effect is bleeding.
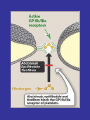
Mechanism of Action of Direct Thrombin Inhibitors as Compared with Heparin
Di Nisio, M. et al. N Engl J Med 2005;353:1028-1040
Synthesis of clotting factors
Oral Anticoagulants
Vitamin K Antagonists (The Coumarins)
• Vitamin K is co-factor for the hepatic
synthesis of clotting factors II, VII, IX & X
warfarin
By Vit.k reductase
Vit. K
Vit. K epoxide (active form)
Warfarin inhibits Vit. K reductase
no active form of Vit. K no
synthesis of clotting factors
ROLE of VITAMIN K
Vitamin K Antagonists (Warfarin)
Onset:
• Clinical anticoagulant activity needs several
days to develop (due to the already circulating
clotting factors)
• So the action of warfarin will appear after the
elimination of prior clotting factors.
• Elimination time (factor II needs: 60 hours,
factor X: 40 hours)
• lasts for 4-5 days
• Overlap heparin & warfarin therapy taken
together until the effect of warfarin appears (after
5 days) then stop taking heparin.
Vitamin K Antagonists
Warfarin
• Warfarin has 100% oral bioavailability
• high plasma protein binding
• Warfarin is metabolized by hepatic Cytochrome
P450 enzymes
• long plasma t1/2 of 36 hours
• A high loading dose followed by an adjusted
maintenance dose
• Warfarin is contraindicated with pregnancy as it
crosses the placental barrier and is teratogenic in the
first trimester & and induce intracranial hemorrhage
in the baby during delivery
• Prothrombin time, a measure of the extrinsic
pathway, may be used to monitor warfarin therapy.
• In the 1990s, the international normalized ratio
(INR) was adopted to monitor warfarin
concentration.
• The INR corrects for variations that would occur
with different thromboplastin reagents, between
different hospitals, or when a single hospital gets a
new lot of reagent.
• The goal of warfarin therapy is an INR of 2 to 3 for
most indications and 2.5 to 3.5 in patients with
mechanical heart valves.
• Warfarin is used to prevent the
progression or recurrence of acute deepvein thrombosis or pulmonary embolism
after initial heparin treatment.
• It is also used for the prevention of
venous
thromboembolism
during
orthopaedic or gynecologic surgery.
• Prophylactically, it is used in patients with
acute myocardial infarction, prosthetic
heart valves, or chronic atrial fibrillation.
Warfarin Drug Pharmacokinetic &
Pharmacodynamic Interactions
Potentiating warfarin
Inhibitors of hepatic P450
enzymes
(cimetidine,
cotrimoxazole,
imipramine,
amiodarone)
Platelet aggregation inhibitors
(NSAIDs e.g. aspirin)
3rd generation cephalosporins*
Drugs displacing warfarin from
binding sites (NSAIDs)
Drugs reducing the availability of
vitamin K
Inhibiting Warfarin
Vitamin K
Inducers of hepatic P450
enzymes
(rifampicin,
barbiturates, … etc)
Reduction of GIT absorption
(cholestyramine)
Diuretics
Hypothyroidism
Hepatic disease(↓ clotting factors) &
hyperthyroidism
*Cephalosporins potentiate warfarin’s effect by
killing vit.k producing normal flora
Warfarin Side-Effects
• Drug-drug interactions
• Bleeding disorder (thus should be
monitored)
Treatment for bleeding
• Minor bleeding: stop therapy + oral
Vitamin K
• Severe Bleeding: stop therapy + I.V.
Vitamin
K
fresh
frozen
plasma,
recombinant factor VIIa or prothrombin
complex may be used
WARFARIN – DRUG INTERACTIONS
Diminished warfarin actions
Ethanol
Enhanced warfarin actions
Ethanol
Aspirin
Cimetidine
Thrombolytics
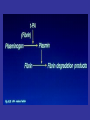
• Fibrinolysis is initiated by tissue plasminogen
activator
(t-PA),
urinary-type
plasminogen
activator (u-PA), and plasmin.
• Plasmin bound to the surface of fibrin initiates the
lysis of insoluble, cross-linked fibrin, with the
subsequent generation of fibrin-degradation
products.
• The main fibrinolytic reactions involve the
inhibition of fibrinolysis by plasminogen-activator
inhibitor type 1 (PAI-1) and {α}2-antiplasmin.
•
The balance between the formation and degradation of FN
Nesheim, M. Chest 2003;124:33S-39S
Thrmobolytics
• Acute
thromboembolic
disease
in
selected patients may be treated by the
administration of agents that activate the
conversion of plasminogen to plasmin
that hydrolyzes fibrin and, thus, dissolves
clots.
• Streptokinase, one of the first such agents
to be approved, causes a systemic
fibrinolytic state that can lead to bleeding
problems.
• Alteplase acts more locally on the
thrombotic fibrin to produce fibrinolysis..
• Clinical experience has shown nearly
equal efficacy betweenn streptokinase and
alteplase..
• Plasmin bound to the surface of fibrin is
better protected from inhibition by {α}2antiplasmin than is plasmin generated in
the fluid phase.
• thrombolytic therapy is unsuccessful in
about 20 % of infarcted arteries, and about
15 % of the arteries that are opened will
later close again.
• In the case of acute myocardial infarction,
the thrombolytic drugs are reserved for
those instances when angioplasty is not
an option or until the patient can be taken
to a facility that performs percutaneous
coronary interventions.
• Clot dissolution and reperfusion occur with
a higher frequency when therapy is
initiated early after clot formation, because
clots become more resistant to lysis as
they age.
• Fibrinolytic drugs may lyse both normal
and pathologic thrombi.
• thrombolytic agents are helpful in restoring
catheter and shunt function, by lysing clots
causing occlusions.
• Thrombolytic agents are also used to dissolve
clots that result in strokes.
• thrombolytic agents are usually administered
intravenously.
• These drugs are contraindicated in patients
with healing wounds, pregnancy, history of
cerebrovascular accident, or metastatic cancer.
streptokinase
• Mechanism of action:
• Streptokinase has no enzymic activity.
Instead, it forms an active one-to-one
complex with plasminogen.
• This
enzymatically
active
complex
converts uncomplexed plasminogen to the
active enzyme plasmin.
• In addition to the hydrolysis of fibrin plugs,
the
complex
also
catalyzes
the
degradation of fibrinogen as well as
clotting Factors V and VII.
Therapeutic uses:
• Streptokinase is approved for use in acute
pulmonary
embolism,
deep-vein
thrombosis, acute myocardial infarction,
arterial thrombosis, and occluded access
shunts.
Pharmacokinetics:
• Streptokinase therapy is instituted within 4
hours of a myocardial infarction and is
infused for 1 hour.
• Its half-life is less than half an hour.
• Thromboplastin time is monitored and
maintained at two- to five-fold the control
value.
• On discontinuation of treatment, either
heparin or oral anticoagulants may be
administered.
•
•
•
•
Hypersensitivity:
Streptokinase is a foreign protein and is antigenic.
Rashes, fever, and rarely, anaphylaxis occur.
Because most individuals have had a streptococcal
infection sometime in their lives, circulating
antibodies against streptokinase are likely to be
present in most patients.
• These antibodies can combine with streptokinase
and neutralize its fibrinolytic properties. Therefore,
sufficient quantities of streptokinase must be
administered to overwhelm the antibodies and
provide a therapeutic concentration of plasmin.
• The incidence of allergic reactions is approximately
3 percent.
alteplase
• Alteplase [AL-te-place] (formerly known as
tissue plasminogen activator, or tPA) is a
serine protease originally derived from
cultured human melanoma cells.
Mechanism of action:
• Alteplase has a low affinity for free
plasminogen in the plasma, but it rapidly
activates plasminogen that is bound to
fibrin in a thrombus or a hemostatic plug.
• Thus, alteplase is said to be fibrin
selective, and at low doses, it has the
advantage of lysing only fibrin, without
unwanted degradation of other proteins
notably fibrinogen.
• This contrasts with streptokinase, which
acts on free plasminogen and induces a
general fibrinolytic state.
• Alteplase seems to be superior to
streptokinase in dissolving older clots.
• Alteplase, administered within 3 hours of
the onset of ischemic stroke, significantly
improves clinical outcome that is, the
patient's ability to perform activities of daily
living.
• Reteplase (Retavase) is similar to
alteplase and can be used as an
alternative.
Anistreplase
streptokinase
complex)
(plasminogen
activator
• Anistreplase is a preformed complex of
streptokinase and plasminogen and it is
considered to be a prodrug.
Agents that control bleeding
Drugs Used to Treat Bleeding
• A. Aminocaproic acid and tranexamic
acid
• Both agents are synthetic, inhibit
plasminogen activation, are orally active,
and are excreted in the urine.
• A potential side effect of treatment is
intravascular thrombosis.
• Protamine sulfate
• antagonizes the anticoagulant effects of
heparin.
• Adverse effects of drug administration
include hypersensitivity as well as dyspnea,
flushing, bradycardia, and hypotension
when rapidly injected.
• Vitamin K
• That
vitamin
K1
(phytonadione)
administration can stop bleeding problems
due to the oral anticoagulants is not
surprising, because those substances act
by interfering with the action of the vitamin.
• The response to vitamin K is slow,
requiring about 24 hours (time to
synthesize new coagulation factors).
• Thus, if immediate hemostasis is required,
fresh-frozen plasma should be infused.
• Aprotinin
• stops bleeding by blocking plasmin.
• It can inhibit streptokinase.
• It is approved for prophylactic use to reduce
perioperative blood loss and the need for blood
transfusion
in
patients
undergoing
cardiopulmonary bypass surgery.
• Aprotinin may cause renal dysfunction and
hypersensitivity (anaphylactic) reactions.
• In addition, aprotinin should not be
administered to patients who have already been
exposed to the drug within the previous 1 year
due to the possibility of anaphylactic reactions.
Anti anaemic Drugs (1).ppt