* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download File
Cracking (chemistry) wikipedia , lookup
Elias James Corey wikipedia , lookup
Petasis reaction wikipedia , lookup
Wolff–Kishner reduction wikipedia , lookup
Kinetic resolution wikipedia , lookup
Strychnine total synthesis wikipedia , lookup
Homoaromaticity wikipedia , lookup
Organosulfur compounds wikipedia , lookup
Asymmetric induction wikipedia , lookup
Hydroformylation wikipedia , lookup
Aromatization wikipedia , lookup
Aromaticity wikipedia , lookup
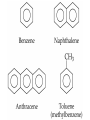
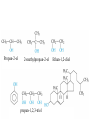
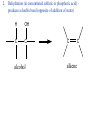
• • • • • Aromatic Hydrocarbons Aromatic structures are formally related to benzene. The delocalized electrons are usually represented as a circle in the center of the ring. Benzene is a planar symmetrical molecule. Benzene is not reactive because of the stability associated with the delocalized electrons. Most aromatic rings are given common names. • Even though they contain bonds, aromatic hydrocarbons undergo substitution more readily than addition (because of delocalization of bonds). • Example: if benzene is treated with nitric acid in the presence of sulfuric acid (catalyst), nitrobenzene is produced. Functional Groups • To get reactivity out of an organic molecule, functional groups have to be added. • Functional groups control how a molecule functions. • More complicated functional groups contain elements other than C or H (heteroatoms). • Functional group containing molecules can either be saturated (alcohols, ethers, amines etc.) or unsaturated (carboxylic acids, esters, amides, etc.). • We usually use R to represent alkyl groups (hydrocarbon). • • • • Alcohols (alkanols) (R-OH) Alcohols are derived from hydrocarbons and contain -OH groups. The names are derived from the hydrocarbon name with ol replacing the e at the end of the -ane suffix. Example: ethane becomes ethanol. Since the O-H bond is polar, alcohols are more water soluble than alkanes. CH3OH, methanol, is used as a gasoline additive and a fuel. • Polyhydroxy alcohols (polyols) contain more than one OH group per molecule (e.g. ethane-1,2-diol - ethylene glycol used as antifreeze). Primary Alcohol – The C on which the OH is attached has only one other C atom directly attached to it. Secondary Alcohol – The C on which the OH is attached has 2 other C atoms directly attached to it. H R C OH R' secondary alchol Tertiary Alcohol – The C on which the OH is attached has 3 other C atoms directly attached to it. R'' R C OH R' tertiary alcohol Naming Alcohols – Name like alkanes (count carbon atoms, attach substituents), replace “e” with “ol.” OH = ethanol Propan-2-ol 2-methylpropan-2-ol propan-1,2,3-triol Ethan-1,2-diol Reactions of Alcohols 1. Complete combustion: C2H5OH + O2 CO2 + H2O 2. Oxidation – warmed with acidified potassium dichromate (Cr2O72-) Primary alcohols oxidized to aldehydes, then to carboxylic acids. O O C C H R C OH R H R OH H primary alcohol aldehyde carboxylic acid To maximize aldehyde production, the product is distilled as it is formed. To maximize carboxylic acid production, the products are heated under reflux for long periods. Secondary alcohols (C with OH is attached to 2 other C) oxidized to ketones H R C O OH C R R' secondary alcohol R' ketone Tertiary alcohols (C with OH is attached to 3 other C – no H atoms attached to C with OH) cannot be oxidized by dichromate 2. Dehydration (in concentrated sulfuric or phosphoric acid) – produces a double bond (opposite of addition of water) H OH C C alcohol C C alkene Ethers (R-O-R′) • Compounds in which two hydrocarbons linked by an oxygen are called ethers. • Ethers are commonly used as solvents. O = ethoxyethane or diethyl ether Compounds with a Carbonyl Group Aldehydes and Ketones • The carbonyl functional group is C=O. • Aldehydes (alkanals) must have at least one H atom O attached to the carbonyl group: R H • Ketones (alkanones) must have two C atoms attached to the carbonyl group: O R R' • Aldehydes and ketones are prepared from the oxidation of alcohols. • Named as alkanes with “-al” (aldehydes) or “-one” (ketones) added to the end. The carbon on which the oxygen is attached is numbered. Examples O 1. propanone O 2. ethanal 3. O heptan-3-one