* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Unsaturated Hydrocarbons I : Alkenes
Cracking (chemistry) wikipedia , lookup
Ring-closing metathesis wikipedia , lookup
Asymmetric induction wikipedia , lookup
Aromaticity wikipedia , lookup
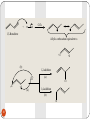
George S. Hammond wikipedia , lookup
Tiffeneau–Demjanov rearrangement wikipedia , lookup
Diels–Alder reaction wikipedia , lookup
Strychnine total synthesis wikipedia , lookup
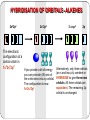
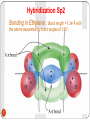
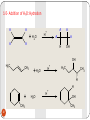
Unsaturated Hydrocarbons 1 Alkenes By: Dr. Siham Lahsasni 1 Unsaturated hydrocarbons that contain one or more double carbon-carbon bonds. Alkenes with just one double C-C bond form an homologous series with the general formula CnH2n. The first few in this series are ethene, propene, butene, and pentene. 2 HYBRIDISATION OF ORBITALS - ALKENES 2s22p2 The electronic configuration of a carbon atom is 1s22s22p2 2s12p3 If you provide a bit of energy you can promote (lift) one of the s electrons into a p orbital. The configuration is now 1s22s12p3 3 x sp2 2p Alternatively, only three orbitals (an s and two p’s) combine or HYBRIDISE to give three new orbitals. All three orbitals are equivalent. The remaining 2p orbital is unchanged. Hybridization Sp2 Bonding in Ethylene : Bond length =1.34 Å with the atoms separated by bond angles of 120°. 4 Nomenclature of alkenes 1. The ene suffix indicates an alkenes or cycloalkenes. 2. The longest chain chosen for the root name must include both carbon atoms of the double bond. 3. The root chain must be numbered from the end nearest a double bond carbon atom. If the double bond is in the center of the chain, the nearest substituent rule is used to determine the end where numbering starts. 4. In cycloalkenes the double bond carbons are assigned ring locations C1 and C2. Which of the two is C1 may be determined by the nearest substituent rule. 5. Substituent groups containing double bonds are: 5 H2C=CH– Vinyl group H2C=CH–CH2– Allyl group CH2 H2C Common IUPAC 6 Ethylene Ethene CH2 H3C Propylene Propene CH2 H2C Br Vinyl bromide Bromoethene H2C Cl Allyl chloride 3-Chloro-1-propene Vinyl cyclohexane Cyclohexyl ethene Alkenyl groups 1. Alkenyl groups are named by adding to alkenes suffix -yl. Ethene becomes a ethenyl group, propene an propenyl group, etc. •Exception case: Methene became Methylene group. 2. Alkenyl groups are named by dropping the -ene suffix of the alkenes and adding the suffix -ylidene. Ethene becomes a ethylidene group, propene an propylidene group, etc. 7 8 Structure and Nomenclature of Dienes Alkenes that contain two double bonds are called Dienes: If the double bonds are separated by only one single bond, the diene is said to be conjugated. If the double bonds are separated by more than one single bond, the diene is called non conjugated diene. Dienes are named by the IUPAC system in essentially the same way as alkenes except that the suffix -adiene replaces the ending –ene of the alkene. Two numbers are needed to indicate the locations of the double bonds in the chain. In cyclic dienes one of the double bonds is always assigned the number 1, and the other is given the lowest possible number. 9 1,4-Pentadiene ( non conjugated) 1,3- Cyclohexadiene (conjugated) 10 1,3-Hexadiene (conjugated) 1,2,3,4-Cyclooctatetraene (conjugated) Geometric (cis-trans) isomerism The prefix cis- is used when the two arms of the longest chain are on the same side of the double bond. The prefix trans- is used when they are on opposite sides of the double bond. 11 E-Z NOTATION FOR GEOMETRIC ISOMERISM If the two groups with the higher molecular weight are on the same side of the double bond, that is described as the (Z)isomer. So you would write it is (Z)-name of compound. If the two groups with the higher molecular weight are on opposite sides of the double bond, then this is the (E)isomer. E 12 Z H 3C C2H5 13 Cl H Br H 3C OH H Physical Properties of Alkenes Alkenes are non polar compounds. Insoluble in water. Soluble in non polar organic solvents. They are less dense than water. Range of physical states: ≤ 4 C's are gases 5 - 17 C's are liquids ≥ 18 C's are solids The alkenes has a boiling point which is a small number of degrees lower than the corresponding alkanes. 14 Preparation of alkenes 1- Dehydration of alcohols: CH 3CH 2OH H2SO 4 / heat or H 3PO4 / Heat Ethanol OH H2C Ethene + H / heat + H2O H cyclohexanol 15 CH2 cyclohexene + H2O Dehydration of a Primary Alcohol: An E2 reaction H+ OH OH 2 E2 H A + HA + H2O Dehydration of a Secondary or tertiary Alcohol: An E1 reaction HSO4 H H H C C H C E1 C OH2 C H C - H2O OH 16 H + C + H2SO4 C H Saytzeff s Rule: In every instance in which more than one alkene can be formed, the major product is the alkene with the most alkyl substituents attached on the double bonded carbon. H2C - H3C CH3 CH3 + H2O 1 Butene minor HO H3C - CH3 2 Butene major 17 + H2O Rearrangement during Dehydration of Secondary Alcohols OH2 OH H+ - H2O rearrangement Secondary carbocation less stable Tertiary carbocation more stable + 18 Minor Major 2- Dehydrohalogenation of Alkyl Halides H2C + KOH CH2 Alcohol H2C heat H X an alkyl halide CH2 + KX + H2O Alkene Br H3C CH3 H KOH alcohol / heat H3C CH3 2-Butene major + H3C CH2 1-Butene minor H Br H CH3 19 + + KOH Alcohol / heat CH3 1-methylcyclohexene major + CH3 3methylcyclohexene minor OH H C C X 20 H E2 C C + H2O + X- 2- Dehydrohalogenation of vicinal dihalides H3C H C H C Cl Cl H 3C H C Br 21 2 NaI CH 3 H C Br acetone H 3C C H C H CH 3 + I2 + CH 3 + ZnBr2 Zn CH 3 CH3COOH or EtOH H 3C C H C H 2 NaCl Reaction of alkenes 1- Additions to the Carbon-Carbon Double Bond 1-1- Addition of hydrogen: Hydrogenation (reduction) A A + A Pt or Ni or Pd H2 A H3C 22 CH2 A A A H An alkene H2C A H An alkane + Pt H2 CH2 + H2 H3C Pt H3C CH3 CH3 1-2- Addition of Halogens: Halogenation A A A + A X2 A A A A X (X= Cl or Br) X Cl H3C CH3 + Cl 2 CCl 4 H3C CH3 Cl Br + 23 Br2 CCl 4 Br Br + Br Br Br Br 24 Br 1-3- Addition of Hydrogen Halides: Hydrohalogenation A A A + A A A HX A A H (X= Cl or Br or I) X Cl H3C CH3 + HCl CCl 4 H3C CH3 H H + HI CCl 4 I 25 H + HX + H X 26 X f ormation of the more stable carbocation Markonikov’s rule: In addition of HX to unsymmetrical alkenes the hydrogen halide adds to the double bonded carbon that bears the greater number of hydrogen atoms and the negative halide ion adds to the other double bonded carbon. H3C H3C H3C 27 CH3 major Br CH2 H3C H3C CH3 + HCl CH 2Br minor H3C H3C CH3 Cl 1-5- Addition of H2O: Hydration A A + A A + H2O H A A A A H OH OH H3C CH3 + + H H2O H3C CH3 H H + + CH3 28 H2O H OH CH3 2- OZONOLYSIS A A A + A A A O3 A H2O, Zn A O A A O O O H3C CH3 1) O 3 H3C 2) H 2O, Zn 1) O 3 H3C CH3 29 2) H 2O, Zn O 2 H3C O + O CH3 + A O A 3- Oxidation KMnO 4 / OH OH - OH 4- Epoxidation H3 O RCOOOH + OH O OH 30 Electrophilic Addition to Conjugated Dienes: 1,4-Addition The addition of a reagent to a pair of adjacent carbons is called 1,2-addition. Br + Br 2 CCl4 1,2-addition 1,4-Pentadiene Br 4,5-Dibromopentene Br Br + Br 2 Br CCl4 1,2-addition Br Br 4,5-Dibromopentene 31 Br 1,2,4,5-Tetrabromopentane Treatement of a conjugated diene with bromine under the similar conditions gives, in addition to the exepected 1,2addition product, an unexcepted 1,4-addition product. 1,2-addition expected product 1,4-addition unexpected product Br + 1,3-Butadiene Br 2 CCl4 + Br 3,4-Dibromobutene 32 Br Br 1,4-Dibromo-2-butene Resonance The addition of bromine to 1,3-butadiene results in a secandary carbocation. This carbocation is also called an allylic carbocation. H 2C C H C H CH 2 H C H 2C Br C H CH 2 H 2C Br Br Br C H CH 2 Allylic carbocation (II) Allylic carbocation (I) H 2C C H Br 1,2-addition Br - 33 resonance H C Br C H Br - 1,4-addition CH 2 H 2C Br C H C H CH 2 Br + H Cl CCl 4 1,3-Butadiene Allylic carbocation equivalent to + (b) 1,2-addition Cl - + + (a) (a) Cl 1,4-addition (b) Cl 34 + The Diels-Alder reaction The reaction is one between a conjugated diene and a compound containing a double bond called a dienophile. The product of a Diels-Alder reaction is offten called an adduct. + Adduct Dienophile Diene O O 100°C + 35 O Benzene O 1,3-Butadiene O Maleic anhydride O