* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download CH 3 CH 2 CH 2 CH 2 CH 2 CH 2 OH
Survey
Document related concepts
Transcript
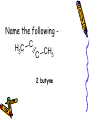
Organic Review organic compound consisting entirely of hydrogen and carbon hydrocarbon Which type of hydrocarbons are saturated Single bonded hydrocarbons Which types of hydrocarbons are unsaturated Double or triple bonded carbons Which are more reactive chemically, saturated hydrocarbons of unsaturated hydrocarbons? unsaturated What is the standard formula of a alkane CnH2n+2 What is the standard formula of a alkene CnH2n What is the standard formula of a alkyne CnH2n-2 What is the ending for a double bonded hydrocarbon? ene What is the ending for a single bonded hydrocarbon? ane What is the ending for a triple bonded hydrocarbon? yne What is the ending for a carboxylic acid? Anoic acid What is the ending for a ketone one What is the ending for an aldehyde? al What is the ending for a ether oxy What is the ending for an ester anoate What is the ending for an alcohol ol What is the beginning for a 7 carbon chain? hept What is the beginning for a 9 carbon chain? non What is the beginning for a 3 carbon chain? prop What is the beginning for a 4 carbon chain? but What is the beginning for a 5 carbon chain? pent What is the beginning for a 2 carbon chain? eth What is the beginning for a 6 carbon chain? hex What is the beginning for a 1 carbon chain? meth What is the beginning for a 8 carbon chain? oct What is the ending for a carboxylic acid? Anoic acid What is the ending for a alcohol? ol What type of reaction will unsaturated hydrocarbons undergo that saturated hydrocarbons will not? Addition reactions . Write a complete balanced equation for the following addition reaction + Cl2 .What type of chemical reaction do all hydrocarbons undergo combustion Write the balanced chemical equation for the complete combustion of ethane Write a complete balanced equation for the following addition reaction + 2 Br2 How does the length of the carbon chain effect the melting points and boiling points of hydrocarbons? The longer the carbon chain the higher the melting and boiling points. Which of the following would you expect to have the highest boiling point? (1) CH3CH2CH2CH2CH3 (2) CH4 (3) CH3CH3 (1) CH3CH2CH2CH2CH3 Are hydrocarbons soluble in water? Why or why not? They are not soluble in water as they are non-polar. What are isomers? Isomers have the same molecular formula but different molecular structure. The double or triple bond will be in a different location Which of the following pairs represents pairs of isomers? H2C H C H2C CH3 H C C H2 H3C CH3 H C CH 2 H3C H C C H CH3 Classify each of the following as an alkane, an alkene or an alkyne - propane alkane Classify each of the following as an alkane, an alkene or an alkyne alkene Classify each of the following as an alkane, an alkene or an alkyne - ethyne alkyne Classify each of the following as an alkane, an alkene or an alkyne alkyne Name the following - 3 - nonyne Name the following - H2C CH2 ethene Name the following C H3C CH 3 C 2 butyne Draw the structural formula and give the molecular formula - butane Draw the structural formula and give the molecular formula - 1 – hexene Draw the structural formula and give the molecular formula - 2 – pentyne Draw the structural formula and give the molecular formula - ethyne Draw the structural formula and give the molecular formula - 4- nonene Why is no number used in the names “ethene” and “propene”? There is only one place you can put the double bond for both molecules. Why is the name 3 - pentyne incorrect? It would be 2 pentyne as you always have to name using the smallest hydrocarbon chain. Describe and explain the relationship between the sizes of hydrocarbon molecules and their boiling points. The longer the hydrocarbon molecule the higher the melting and boiling point. is a method used to separate the components of petroleum It involves the process of distillation, making use of the differences in boiling points of the different hydrocarbons. fractional distillation To make fuels better for internal combustion engines straight chain hydrocarbons are broken down into shorter branched chain hydrocarbons process of cracking a particular combination of atoms that contributes to the physical and chemical characteristics of a substance functional group What type of functional group does an alcohol contain OH (hydroxyl) group Give the physical properties of alcohols • polar • higher melting and boiling points the alkanes of similar chain length • longer the carbon chain is the higher the boiling point will be Write the equation for the complete combustion of methanol. 2CH3OH + 3O2 ===> 2CO2 + 4H2O Name the - CH3CH2OH ethanol Name - CH3CH2CH(OH)CH3 2 - butenol Name - CH3 CH2 CH2CH(OH) CH2 CH2CH3 4 - heptanol Draw the structural formula for - 1 – propanol Draw the structural formula for - ethanol Draw the structural formula for –, 1 - hexanol What type of functional group does an ether contain? R – O - R . Explain why methanol has a higher melting point than methane. It contains an hydroxyl so is polar and forms hydrogen bonds and will therefore take more energy to split the bonds thus causing an higher melting point. Arrange the following compounds in order of increasing boiling point. 1 – pentanol, decane, 1 – decanol, pentane Pentane, 1-pentanol,decane, decanol Consider the following two compounds: CH3CH2CH2CH2CH2CH2OH and CH3CH2CH2OCH2CH2CH3. Which is an ether and which is an alcohol • CH3CH2CH2CH2CH2CH2OH – alcohol • CH3CH2CH2OCH2CH2CH3. - ether Which will evaporate at the lower temperature CH3CH2CH2CH2CH2CH2OH or CH3CH2CH2OCH2CH2CH3. CH3CH2CH2OCH2CH2CH3. - ether Which has the higher solubility in a polar solvent CH3CH2CH2CH2CH2CH2OH or CH3CH2CH2OCH2CH2CH3. CH3CH2CH2CH2CH2CH2OH – alcohol What type of functional group does a carboxylic acid have? COOH What type of chemical reaction is common for carboxylic acids Esterfication and neutralization reactions. What is the functional group for an ester? What are the products and the reactants in an esterification reaction? Alcohols + Carboxylic acid -- ester . What is the most distinctive property of esters? The have a very strong odor. What are esters commonly used for? Cleaning solutions What is a hydrolysis reaction? Using water to break down a compounds into smaller parts. What is the functional group for ketones What is the functional group for aldehydes Draw the structure of butanal. Draw the structure for 2pentanone. What is a plastic? synthetic (man made) polymer What are large molecules that are formed by linking many small molecules together called: polymer A molecule that is able to bond in long chains. monomer What is the difference between an addition polymer and a condensation polymer? Give an example of each addition polymers the monomers will contain one or more multiple bonds that can undergo addition reactions. condensation reaction water is a product