* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Reactions of Alcohols
Discodermolide wikipedia , lookup
Elias James Corey wikipedia , lookup
Kinetic resolution wikipedia , lookup
Asymmetric induction wikipedia , lookup
Petasis reaction wikipedia , lookup
Wolff rearrangement wikipedia , lookup
Hydroformylation wikipedia , lookup
Strychnine total synthesis wikipedia , lookup
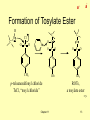
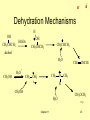
Organic Chemistry, 6th Edition L. G. Wade, Jr. Chapter 11 Reactions of Alcohols _ + _ + Types of Alcohol Reactions • • • • • • • Dehydration to alkene Oxidation to aldehyde, ketone Substitution to form alkyl halide Reduction to alkane Esterification Tosylation Williamson synthesis of ether Chapter 11 => 2 _ + Summary Table Chapter 11 3 => _ + Oxidation States • Easy for inorganic salts CrO42- reduced to Cr2O3 KMnO4 reduced to MnO2 • Oxidation: loss of H2, gain of O, O2, or X2 • Reduction: gain of H2 or H-, loss of O, O2, or X2 • Neither: gain or loss of H+, H2O, HX => Chapter 11 4 _ + 1º, 2º, 3º Carbons => Chapter 11 5 _ + Oxidation of 2° Alcohols • • • • 2° alcohol becomes a ketone Reagent is Na2Cr2O7/H2SO4 Active reagent probably H2CrO4 Color change: orange to greenish-blue OH CH3CHCH2CH3 Na2Cr2O7 / H2SO4 O CH3CCH2CH3 => Chapter 11 6 _ + Oxidation of 1° Alcohols • 1° alcohol to aldehyde to carboxylic acid • Difficult to stop at aldehyde • Use pyridinium chlorochromate (PCC) to limit the oxidation. • PCC can also be used to oxidize 2° alcohols to ketones. OH N H CrO3Cl CH3CH2CH2CH2 O CH3CH2CH2CH => Chapter 11 7 _ + 3° Alcohols Don’t Oxidize • Cannot lose 2 H’s • Basis for chromic acid test Chapter 11 => 8 _ + Other Oxidation Reagents • • • • • • Collins reagent: Cr2O3 in pyridine Jones reagent: chromic acid in acetone KMnO4 (strong oxidizer) Nitric acid (strong oxidizer) CuO, 300°C (industrial dehydrogenation) Swern oxidation: dimethylsulfoxide, with oxalyl chloride and hindered base, oxidizes 2 alcohols to ketones and 1 alcohols to aldehydes. => Chapter 11 9 Biological Oxidation _ + • Catalyzed by ADH, alcohol dehydrogenase. • Oxidizing agent is NAD+, nicotinamide adenine dinucleotide. • Ethanol oxidizes to acetaldehyde, then acetic acid, a normal metabolite. • Methanol oxidizes to formaldehyde, then formic acid, more toxic than methanol. • Ethylene glycol oxidizes to oxalic acid, toxic. • Treatment for poisoning is excess ethanol. => Chapter 11 10 _ + Alcohol as a Nucleophile H C O R X • ROH is weak nucleophile • RO- is strong nucleophile • New O-C bond forms, O-H bond breaks. => Chapter 11 11 _ + Alcohol as an Electrophile • OH- is not a good leaving group unless it is protonated, but most nucleophiles are strong bases which would remove H+. • Convert to tosylate (good leaving group) to react with strong nucleophile (base). H + C O C-Nuc bond forms, C-O bond breaks => Chapter 11 12 _ + Formation of Tosylate Ester H C O C C H O O Cl O S O N O CH3 S O CH3 p-toluenesulfonyl chloride TsCl, “tosyl chloride” Chapter 11 O S O CH3 ROTs, a tosylate ester => 13 _ + SN2 Reactions of Tosylates • • • • • • With hydroxide produces alcohol With cyanide produces nitrile With halide ion produces alkyl halide With alkoxide ion produces ether With ammonia produces amine salt With LiAlH4 produces alkane => Chapter 11 14 _ + Summary of Tosylate Reactions => Chapter 11 15 _ + Reaction with HBr • • • • -OH of alcohol is protonated -OH2+ is good leaving group 3° and 2° alcohols react with Br via SN1 1° alcohols react via SN2 R O H H3O + H R O H Chapter 11 - Br R Br => 16 Reaction with HCl _ + • Chloride is a weaker nucleophile than bromide. • Add ZnCl2, which bonds strongly with -OH, to promote the reaction. • The chloride product is insoluble. • Lucas test: ZnCl2 in conc. HCl 1° alcohols react slowly or not at all. 2 alcohols react in 1-5 minutes. 3 alcohols react in less than 1 minute. => Chapter 11 17 _ + Limitations of HX Reactions • • • • HI does not react Poor yields of 1° and 2° chlorides May get alkene instead of alkyl halide Carbocation intermediate may rearrange. => Chapter 11 18 _ + Reactions with Phosphorus Halides • • • • Good yields with 1° and 2° alcohols PCl3 for alkyl chloride (but SOCl2 better) PBr3 for alkyl bromide P and I2 for alkyl iodide (PI3 not stable) => Chapter 11 19 Mechanism with PBr3 _ + • P bonds to -OH as Br leaves • Br- attacks backside (S 2) N • HOPBr2 leaves Chapter 11 => 20 Reaction with Thionyl Chloride • • • • _ + Produces alkyl chloride, SO2, HCl S bonds to -OH, Cl leaves Cl- abstracts H+ from OH C-O bond breaks as Cl transferred to C Chapter 11 21 => _ + Dehydration Reactions • • • • • • Conc. H2SO4 produces alkene Carbocation intermediate Zaitsev product Bimolecular dehydration produces ether Low temp, 140°C and below, favors ether High temp, 180°C and above, favors alkene => Chapter 11 22 _ + Dehydration Mechanisms H OH CH3CHCH3 H2SO4 OH CH3CHCH3 CH3CHCH3 alcohol H2O CH3OH H3O CH2 CHCH3 + CH3 CH3 OH2 O CH3 H CH3OH H2O Chapter 11 CH3OCH3 => 23 Energy Diagram, E1 _ + => Chapter 11 24 _ + Alkoxide Ions • ROH + Na (or NaH) yields sodium alkoxide • RO- + 1° alkyl halide yields ether (Williamson ether synthesis) CH3 CH3CH2CHCH3 + CH3CH2 Br O CH2CH2CH O CH2CH3 => Chapter 11 25 _ + End of Chapter 11 Chapter 11 26