* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Carey Chapter 4 Alcohols, Alkyl Halides
Elias James Corey wikipedia , lookup
George S. Hammond wikipedia , lookup
Hofmann–Löffler reaction wikipedia , lookup
Petasis reaction wikipedia , lookup
Kinetic resolution wikipedia , lookup
Tiffeneau–Demjanov rearrangement wikipedia , lookup
Strychnine total synthesis wikipedia , lookup
Hydroformylation wikipedia , lookup
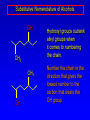
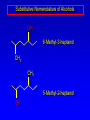
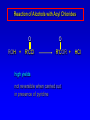
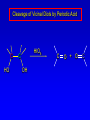
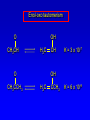
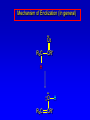
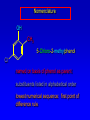
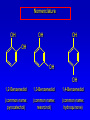
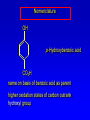
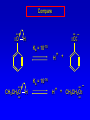
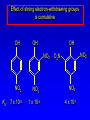
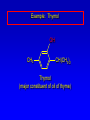
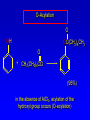
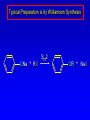
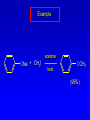
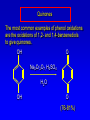
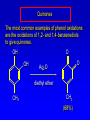
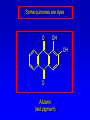
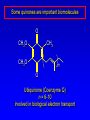
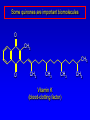
Alcohols, enols and phenols Classification Alcohols and alkyl halides are classified as primary secondary tertiary according to their "degree of substitution." Degree of substitution is determined by counting the number of carbon atoms directly attached to the carbon that bears the halogen or hydroxyl group. Classification H CH3CH2CH2CH2CH2F OH primary alkyl halide secondary alcohol CH3 CH3CHCH2CH2CH3 Br secondary alkyl halide CH3CCH2CH2CH3 OH tertiary alcohol IUPAC Nomenclature of Alcohols Functional Class Nomenclature of Alcohols Name the alkyl group and add "alcohol" as a separate word. CH3CH2OH CH3 CH3CCH2CH2CH3 CH3CHCH2CH2CH2CH3 OH OH Functional Class Nomenclature of Alcohols Name the alkyl group and add "alcohol" as a separate word. CH3CH2OH Ethyl alcohol CH3CHCH2CH2CH2CH3 OH 1-Methylpentyl alcohol CH3 CH3CCH2CH2CH3 OH 1,1-Dimethylbutyl alcohol Substitutive Nomenclature of Alcohols Name as "alkanols." Replace -e ending of alkane name by -ol. Number chain in direction that gives lowest number to the carbon that bears the —OH group. CH3CH2OH CH3 CH3CCH2CH2CH3 CH3CHCH2CH2CH2CH3 OH OH Substitutive Nomenclature of Alcohols Name as "alkanols." Replace -e ending of alkane name by -ol. Number chain in direction that gives lowest number to the carbon that bears the —OH group. CH3CH2OH Ethanol CH3CHCH2CH2CH2CH3 OH CH3 CH3CCH2CH2CH3 OH 2-Methyl-2-pentanol 2-Hexanol Substitutive Nomenclature of Alcohols OH CH3 CH3 OH Hydroxyl groups outrank alkyl groups when it comes to numbering the chain. Number the chain in the direction that gives the lowest number to the carbon that bears the OH group Substitutive Nomenclature of Alcohols OH 6-Methyl-3-heptanol CH3 CH3 5-Methyl-2-heptanol OH Dipole Moments alcohols and alkyl halides are polar = 1.7 D = 1.9 D Effect of Structure on Boiling Point CH3CH2OH Molecular weight 46 Boiling point, °C +78 Dipole moment, D 1.7 Highest boiling point; strongest intermolecular attractive forces. Hydrogen bonding is stronger than other dipole-dipole attractions. Hydrogen bonding in ethanol Reaction of Alcohols with Acyl Chlorides O ROH + R'CCl O R'COR + high yields not reversible when carried out in presence of pyridine HCl Esterification O ROH + H+ O R'COR + R'COH a condensation reaction called Fischer esterification acid catalyzed reversible H2O Reaction of Alcohols with Acid Anhydrides O O ROH + R'COCR' O R'COR + analogous to reaction with acyl chlorides O R'COH Esters of Inorganic Acids ROH + HOEWG ROEWG + H2O EWG is an electron-withdrawing group + HONO2 (HO)2SO2 (HO)3P – O Oxidation of Alcohols Primary alcohols O O RCH2OH RCH RCOH Secondary alcohols OH O RCHR' RCR' from H2O Enzyme-catalyzed CH3CH2OH + NAD + (a coenzyme) alcohol dehydrogenase CH3CH O + NAD H + H+ Cleavage of Vicinal Diols by Periodic Acid C HO HIO4 C OH C O + O C Enols and Enolates Enol-oxo tautomerism OH O CH3CH H2C K = 3 x 10-7 OH O CH3CCH3 CH H2C CCH3 K = 6 x 10-9 Mechanism of Enolization (In general) •• O •• R2C CR' H •• •• O R2C CR' H Enol Content OH O R2CHCR' R2C keto CR' enol percent enol is usually very small keto form usually 45-60 kJ/mol more stable than enol Phenols Nomenclature OH CH3 5-Chloro-2-methylphenol Cl named on basis of phenol as parent substituents listed in alphabetical order lowest numerical sequence: first point of difference rule Nomenclature OH OH OH OH OH OH 1,2-Benzenediol 1,3-Benzenediol 1,4-Benzenediol (common name: pyrocatechol) (common name: resorcinol) (common name: hydroquinone) Nomenclature OH p-Hydroxybenzoic acid CO2H name on basis of benzoic acid as parent higher oxidation states of carbon outrank hydroxyl group Structure of Phenol phenol is planar C—O bond distance is 136 pm, which is slightly shorter than that of CH3OH (142 pm) Physical Properties The OH group of phenols allows hydrogen bonding to other phenol molecules and to water. Hydrogen Bonding in Phenols O H O Acidity of Phenols most characteristic property of phenols is their acidity Compare •• •• O •• – •• O •• H Ka = 10-10 •• CH3CH2O •• + + H Ka = 10-16 H + + H •• – CH3CH2O •• •• Effect of strong electron-withdrawing groups is cumulative OH OH OH NO2 NO2 NO2 Ka: 7 x 10-8 1 x 10-4 NO2 O2N NO2 4 x 10-1 Example: Thymol OH CH3 CH(CH3)2 Thymol (major constituent of oil of thyme) Example: 2,5-Dichlorophenol OH Cl Cl 2,5-Dichlorophenol (from defensive secretion of a species of grasshopper) O-Acylation O OH OC(CH2)6CH3 O + CH3(CH2)6CCl (95%) in the absence of AlCl3, acylation of the hydroxyl group occurs (O-acylation) Typical Preparation is by Williamson Synthesis ONa + RX SN2 OR + NaX Example acetone ONa + CH3I heat OCH3 (95%) Quinones The most common examples of phenol oxidations are the oxidations of 1,2- and 1,4-benzenediols to give quinones. OH O Na2Cr2O7, H2SO4 H2O OH O (76-81%) Quinones The most common examples of phenol oxidations are the oxidations of 1,2- and 1,4-benzenediols to give quinones. OH O OH O Ag2O diethyl ether CH3 CH3 (68%) Some quinones are dyes O OH OH O Alizarin (red pigment) Some quinones are important biomolecules O CH3 CH3O CH3O n O Ubiquinone (Coenzyme Q) n = 6-10 involved in biological electron transport Some quinones are important biomolecules O CH3 CH3 O CH3 CH3 CH3 Vitamin K (blood-clotting factor) CH3