* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Ch 17- Aldehydes and Ketones
Ring-closing metathesis wikipedia , lookup
Homoaromaticity wikipedia , lookup
Aromaticity wikipedia , lookup
Aldol reaction wikipedia , lookup
1,3-Dipolar cycloaddition wikipedia , lookup
Metal carbonyl wikipedia , lookup
Strychnine total synthesis wikipedia , lookup
Wolff–Kishner reduction wikipedia , lookup
Carbohydrate wikipedia , lookup
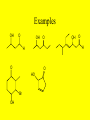
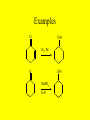
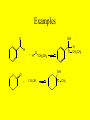
Ch 17- Aldehydes and Ketones Homework: 17.10, 17.13, 17.17, 17.19, 17.28, 17.35, 17.40, 17.41, 17.49, 17.53, 17.69 • In this chapter we study compounds that contain the carbonyl group. O C The Carbonyl group • The carbonyl group is one of the most important functional groups in organic chemistry and Biochemistry because it is present in aldehydes, ketones, carboxylic acid and carboxylic acid derivatives. Aldehydes & Ketones • The functional group of an aldehyde is a carbonyl group bonded to a hydrogen • In methanal, the simplest aldehyde, the carbonyl group is bonded to 2 H’s • In all other aldehydes, it is bonded to one H and a carbon chain. • The functional group of a ketone is a carbonyl group bonded to 2 carbon chains. Nomenclature • 1) Find the longest chain that contains the carbon of the carbonyl and number the chain to give that carbon the lowest number. – For aldehydes, drop the -e, and add -al – Aldehydes will always be at the end of a chain, so they will always be at carbon #1, so there is no need to put the 1. O H O H O H Nomenclature (cont) • For aldehydes that have carbon-carbon double bonds in them, the longest chain must contain the carbon of the carbonyl and BOTH carbons of the C-C double bond!! • To name these, we drop the -ane ending and add -enal • The -en- shows the double bond, the -al shows the aldehyde. – Remember to provide the locant for the double bond O H O H O H Nomenclature of Ketones • For ketones, we find the longest chain that contains the carbon of the carbonyl group, and number the chain to give that carbon the lowest number • You drop the -e, from the parent name and add -one. O O O • In naming aldehydes and ketones that also have an -OH in the molecule, find the longest chain that contains both the carbon of the carbonyl and the carbon bonded to the -OH group • Number the chain to give the carbon of the carbonyl the lowest number • The -OH will be named as a substituent! • When the -OH group is named as a substituent, it is named as a hydroxy, and numbered and alphabetized will all other substituents present. Examples OH O OH O O O HO OH O H H Br OH Physical Properties • Oxygen is more electronegative than carbon therefore the carbon-oxygen double bond is polar with the Oxygen bearing a partial negative charge and the Carbon bearing a partial positive charge • The only intermolecular forces are dipoledipole forces and London Forces • They can not Hydrogen bond to each other!! Physical Properties • As the groups bonded to the carbonyl increase in size, the solubility in water decreases • Most aldehydes and ketones have strong odors and are used in perfumes and flavoring agents Reactions 1) Aldehydes can be oxidized to the carboxylic acids O O K2Cr2O7 OH H2SO4 H -Ketones are resistant to oxidation Aldehydes can also be oxidized by O2!! O O H O2 OH Reduction Reactions • Just like C-C double bonds, C-O double bonds can be reduced by the addition of H2 with a metal catalyst to produce an alcohol. OH O H2 H O Pd, Pt, or Ni H2 Pd, Pt, or Ni OH Sodium Borohydride • Aldehydes and Ketones can also be reduced using Sodium Borohydride, NaBH4 • NaBH4 contains hydrogen in the form of hydride ions, H- • The advantage of using NaBH4 is that it does not reduce C-C double bonds! Examples O OH H2, Pd O OH NaBH4 H3O In Nature • In Biological systems, nicotinamide adenine dinucleotide, a coenzyme abbreviated NADH, is used to provide the hydride ion to reduce aldehydes and ketones. O H3C O NADH COO Pyruvate H3C H OH H3O COO H3C H Lactate COO Reactions of Alcohols 3) Addition of Alcohols Addition of one molecule on an alcohol to an aldehyde or ketone form a hemiacetal Hemiacetal- a compound with a carbon bonded to 1 -OH group and 1 -OR group Examples O OH H O + H O CH CH 2 3 OH O + CH3 OH O CH3 H CH2CH3 Reactions of Hemiacetals • Hemiacetals can react with another molecule of alcohol to form an acetal • Acetal- a compound with a carbon bonded to 2 -OR groups OH O O H CH2CH3 + O H CH2CH3 OH O CH3 O O + CH3 OH CH2CH3 CH3 O CH3 H CH2CH3 Info on Hemiacetals • Hemiacetals are generally unstable and are only minor components of an equilibrium mixture • The only exception is when the -OH group is part of the same molecule as the carbonyl group and a five or six member ring can form • The compound exists almost entirely in the cyclic hemiacetal forms • In this case, the -OH group adds to the C=O group of the same molecule Examples 3 O 5 4 3 2 1 Redraw to show -OH and carbonyl H 5 2 1 4 OH close to each other HO 4-Hydroxypentanal 3 5 2 1 4 OH H O 3 4 5 O 2 1 H OH A Cyclic Hemiacetal H O YOUR TURN!! • Do the same thing with: O OH H OH OH OH OH NOTE: Six membered rings are more stable than five membered Rings. If both can form, the six membered ring will form over the Five membered ring Keto-Enol Tautomerism • A carbon atom adjacent to a carbonyl group is called an a-carbon, and a hydrogen atom bonded to it is called an a-hydrogen Alpha Carbons O C H3C CH3 C H2 Alpha Hydrogens Beta Carbon ( Keto-Enol Tautomerism • A carbonyl compound that has an ahydrogen is in equilibrium with a constitutional isomer called an enol • The name enol is derived from the IUPAC designation of it as having both an alkene (-en) and an alcohol (-ol) OH O H3C CH3 H3C CH2 Keto-Enol Tautomerism • The Keto and Enol forms are examples of Tautomers. • Tautomer- constitutional isomers in equilibrium with each other that differ in the location of a hydrogen atom relative to an oxygen atom • This type of isomerism is called keto-enol tautomerism • For any pair of keto-enol tautomers, the keto form generally predominates at equilibrium!