* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Introduction to Human Physiology
Biochemical cascade wikipedia , lookup
Cell culture wikipedia , lookup
Cell growth wikipedia , lookup
Evolution of metal ions in biological systems wikipedia , lookup
Artificial cell wikipedia , lookup
Polyclonal B cell response wikipedia , lookup
Vectors in gene therapy wikipedia , lookup
Developmental biology wikipedia , lookup
Cell (biology) wikipedia , lookup
Signal transduction wikipedia , lookup
Organ-on-a-chip wikipedia , lookup
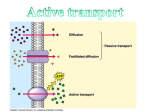
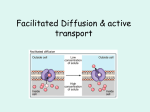
Introduction to Human Physiology What is physiology? It is the science that deals with the function of organs and systems and the way they do this functions and the way they integrate. Exams and marks 250 marks: 125 final exam 45 practical 30 oral 40 midterm 10 shock exam Man and the environment External environment: (variable surroundings) The environment man lives in, interacts with (benefits and hazards) Major environmental factors are: oxygen, water, food, physical factors, social factors, micro organisms and parasites. Body-Fluid Compartments H20: 60% of TB.Wt. in adult male. 75% of TB.Wt in infants Less than 60% in adult female and obese people TBW is disributed as: Intracellular compartment (ICF) Extracellular compartment (ECF) Fluid inside the cells Fluid outside the cells 2/3 of TBW. 1/3 of TBW 40% of TB.Wt 20% of TB.Wt 2 Subdivisions: Blood plasma IVF (5%) Interstitial fluid ISF (15%) Body water Interstitial fluid=15% Stomach& intestine Cell water= 40% Intravascular fluid=5% lungs Capillary wall Kidneys &skin The concentration of the minerals in the intracellular fluid is different from the concentration of them in the extra cellular fluid. The total blood volume is 8% of TBWt Intracellular fluid Extra cellular fluid ICF Plasma ISF Na K 10 155 145 4.5 150 4.0 Ca Mg 0.001 13 2.5 1 1.5 1 Cl HCO3 3 10 115 28 110 27 HPO3 Glucose 50 1 1 4-6 1 4-6 Proteins ratio 4 1 O-1 Osmolality Osmol/L 300 300 300 CATIONS mmol/L ANIONS mmol/L Determination of the volumes of water in the body Fick`s principle Indicator: inert, non toxic, not utilized by tissues. Known amount of indicator is injected intravenously 9gm Time is allowed for uniform diffusion A sample is withdrawn to determine the concentration in the plasma 3gm/L Volume of the compartment (volume of the distribution) = amount injected / concentration in plasma 9 gm/3gm/L= 3L Application: 1- TBW: Heavy water (D2O) deuterium oxide 2- ECF: Inulin (polysaccharide fiber) or Na thiocyanate (NaSCN) 3- Plasma: Evan`s blue or radioactive plasma proteins 4- ICF: TBW- ECF 5-ISF: ECF-Plasma volume Homeostasis The internal environment is the interstitial fluid that surrounds the cells The internal environment The human body consists of organs& tissues that are formed of cells. The cell is the smallest unit of life. The cell is surrounded by interstitial fluid (Internal Environment). The composition of the internal environment should remain constant within narrow limits. Internal environment All the life processes have only one goal, that is to keep the internal environment constant, and this fixity of the internal environment is necessary condition for life Homeostasis: It is all the physiological processes that are carried out by all body systems. It is to insure that chemical and physical structure of the internal environment is kept constant in spite of external (e.g.: temp, oxygen tension, pressure) or internal (e.g.: increased muscular activity) changes. It deals with all automatic reactions which take place to correct all deviations from normal It is a necessary condition for the existence Failure of homeostasis often leads to diseases and death. Body water Interstitial fluid=15% Stomach& intestine Cell water= 40% Intravascular fluid=5% lungs Capillary wall Kidneys &skin Cell membrane ICF ISF plasma organs internal environment external environment Exchange and communication are key concepts for understanding physiological homeostasis. Homeostasis: The body’s ability to maintain a stable internal environment. Toxic Chemicals Trauma Viruses Bacteria Cancer Autoimmune Disease Sickle cell anemia Diabetes Example for homeostasis Cells consumes glucose & O2 Cells takes glucose & O2 from ISF (1st exchange through cell membrane) ISF takes these substances from blood (2nd exchange through capillary wall) Blood brings new nutrients & O2 from systems from external environment Cell Unit of function of living organism Cells with similar properties….… tissue. Different tissues……organs. Complementary organs…..systems The structure of the cell varies according to the function (specialization) No typical cell All cells are formed of mass of protoplasm surrounded by cell membrane The Cell Membrane *Very thin (7.5-10 nm) *Elastic *Semipermeable * Dynamic * EM: lipid bilayer & proteins *Made of proteins 55%, phospholipids 25%, cholesterol 13%, other lipids 4% &CHO 3%, *amphipathic (hydrophilic & hydrophobic) Phospholipids Flexibility & selective permeability Fatty acid tails – hydrophobic Phosphate Phosphate group head – hydrophilic Arranged as a bilayer Fatty acid Phospholipid bilayer polar hydrophilic heads nonpolar hydrophobic tails polar hydrophilic heads Protein: *Hydrophilic & hydrophobic *Integral & peripheral Cholesterol: permeability & toughness CHO: recognition sites & attach cells Glycoprotein Glycolipid together Cholesterol Peripheral protein Functions of membrane proteins 1- structural ptns 2- passive channels: ungated & gated(voltage / ligand) 3- carriers for facilitated diffusion 4- carriers for active transport (uniport /symport/ antiport) 5- receptors: number & sensitivity change 6- enzymes 7- identity ptns 8-intercellular connections: a. binding j: tight & desmosomes b. gap j 9- cell adhesion molecules 10- fixation of cytoskeleton Many Functions of Membrane Proteins Outside Plasma membrane Inside Transporter Enzyme activity Cell surface receptor Cell surface identity marker Cell adhesion Attachment to the cytoskeleton Intercellular connections binding and channel junctions Gap junctions: for rapid propagation of electrical activity allow rapid passage of ions and molecules up to MW1000 diameter is regulated by Ca, pH, hormones and drugs Membrane carbohydrates Play a key role in cell-cell recognition – ability of a cell to distinguish one cell from another • antigens – important in organ & tissue development – basis for rejection of foreign cells by immune system Movement across the Cell Membrane 2007-2008 Diffusion All molecules are in constant motion Diffusion: Passive movement from high low concentration Diffusion across cell membrane 1- simple diffusion: with concentration gradient- no energy- no carrier Diffusion rate α con gradient x surface area x temp /√mol wt x distance 1- through lipid bilayer: Lipid soluble sub Water Lipid insoluble sub (urea) 2- through protein channels: Ions electrically charged hydrated Each protein channel is specific through diameter, shape, electrical charge & gates Selective permeability gating 2- facilitated diffusion: with conc. gradient, passive, carrier for large molecules Characters: Specificity competition rate increases with concentration gradient up to maximum more sensitive to temperature AS Biology, Cell membranes and Transport 34 Facilitated Diffusion Diffusion through protein channels – channels move specific molecules across cell membrane facilitated = with help – no energy needed open channel = fast transport high low Osmosis is diffusion of water Diffusion of water from high concentration of water to low concentration of water – across a semi-permeable membrane – The pressure necessary to stop solvent mol movements= osmotic pressure – The numbers of particles per unit volume of fluid – Measured in mmHg – Osmole osmolarity osmolality The osmolarity of ICF=that ECF=300 mosmol 280 mosmol is due to Na, Cl & HCO3 20 mosmol is due to protein Tonicity: is the osmolality of a solution relative to the plasma Plasma proteins of blood is called oncotic pressure. It is important for capillary circulation ®ulation of ECF Concentration of water Direction of osmosis is determined by comparing total solute concentrations – Hypertonic - more solute, less water – Hypotonic - less solute, more water – Isotonic - equal solute, equal water water hypotonic hypertonic net movement of water Donnan effect The protein anions inside the cells are non diffusible hinder the diffusion of diffusible cations More osmotically active particles inside the cell The cell tends to swell But the Na+/ K+ pump prevents cell rupture Donnan effect 4K+ 4Cl- 4K+ 2Cl- & 2 Ptn- 3Cl3K+ 3Cl-& 2Ptn5K+ 3. Gibbs-Donnan Equilibrium - YouTube.flv Active Transport Against concentration gradient Needs carrier protein Energy is needed low conformational change ATP high “The Doorman” Active transport Many models & mechanisms ATP ATP antiport symport Active transport 1ry active: *eg Na+/K+ pump *α &β subunits *α subunit contains 2 binding sites for K+ on the outside & 3 binding sites for Na+ on the inside & an ATP binding site *β subunit has ATPase activity. 2ry active: *eg Glucose transport 2ry to active transport of Na 1st Na pumped out ….creates concentration gradient… Na & glucose bind a carrier…transports them to inside Figure 5.13 Primary Active Transport: The Sodium–Potassium Pump In active transport, energy is used to move a solute against its concentration gradient. For each molecule of ATP used, 2 K+ are pumped into the cell and 3 Na+ are pumped out of the cell. K+ Outside of cell Sodium– potassium pump Inside of cell Na+ Getting through cell membrane Passive Transport Simple diffusion diffusion of nonpolar, hydrophobic molecules lipids high low concentration gradient Facilitated transport diffusion of polar, hydrophilic molecules through a protein carrier high low concentration gradient Active transport diffusion against concentration gradient low high uses a protein pump requires ATP Vesicular transport ATP Transport summary simple diffusion facilitated diffusion active transport ATP How about large molecules? Moving large molecules into & out of cell – through vesicles & vacuoles – endocytosis • phagocytosis = “cellular eating” • pinocytosis = “cellular drinking” – exocytosis exocytosis Endocytosis phagocytosis fuse with lysosome for digestion pinocytosis non-specific process receptor-mediated endocytosis triggered by molecular signal Exocytosis The opposite of endocytosis is exocytosis. Large molecules that are manufactured in the cell are released through the cell membrane. AS Biology, Cell membranes and Transport 53 These are carrier proteins. They do not extend through the membrane. They bond and drag molecules through the bilipid layer and release them on the opposite side. AS Biology, Cell membranes and Transport 54 Vesicle-mediated transport Vesicles and vacuoles that fuse with the cell membrane may be utilized to release or transport chemicals out of the cell or to allow them to enter a cell. Exocytosis is the term applied when transport is out of the cell. 55 Cell Membrane - Function - Endocytosis The cell membrane can also engulf structures that are much too large to fit through the pores in the membrane proteins this process is known as endocytosis. In this process the membrane itself wraps around the particle and pinches off a vesicle inside the cell. In this animation an ameba engulfs a food particle. 56 Clatherin mediated endocytosis Endocytosis and Exocytosis Endocytosis Any Questions?? Quiz i. Explain the transport function of the plasma membrane protein ii. 1- Glucose transport into the intestinal cells can be increased by: a. increase temperature b. increase galactose concentration c. increase concentration gradient for glucose without limits d. increase the thickness of the cell membrane 2- Concerning the body water compartments: a. the intravascular compartment is the largest compartment b. the interstitial fluid is the internal environment c. Ca2+ is the main cation intracellularly d. protein is more in the interstitial fluid than in the plasma 3- Inulin is used for direct measuring the volume of: a. ECF b. IVF c. total body water d. plasma volume Endocytosis and Exocytosis Receptor Proteins These proteins are used in intercellular communication. In this animation you can see the a hormone binding to the receptor. This causes the receptor protein release a signal to perform some action. 64 Intracellular communications A] 3 main types of communication: 1- intercellular gap junctions 2- neural neurotransmitters 3- endocrine communication, paracrine, autocrine B] messengers bind receptors C] intracellular effects through: 1- opening channels 2- activation of adenyl cyclase 3- increase free intracellular Ca2+ Intercellular communications Gap Junctions Synaptic Paracrine & Autocrine Directly from cell to cell Across synaptic cleft By diffusion in interstitial fluid By circulating body fluids Local Local Local diffusion Systemic Endocrine Regulating systems 1- endocrine system: slow prolonged effect 2- nervous system: rapid short effect The neuron The basic structural unit of the nervous system. Structure: The soma The dendrites: antenna like processes The axon: hillock, terminal buttons Types of nerve fibers a- myelinated nerve fiber: Covered by myelin sheath b- unmyelinated nerve fiber: Myelin sheath is absent Synapses: it is the site where the axon of one neuron ends & the dendrites of another begins There is space called synaptic cleft Chemical transmitters are released at synapses