* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Inflammation Regulation Drug Traumeel
Survey
Document related concepts
Immune system wikipedia , lookup
Lymphopoiesis wikipedia , lookup
Molecular mimicry wikipedia , lookup
Cancer immunotherapy wikipedia , lookup
Rheumatoid arthritis wikipedia , lookup
Adaptive immune system wikipedia , lookup
Sjögren syndrome wikipedia , lookup
Polyclonal B cell response wikipedia , lookup
Innate immune system wikipedia , lookup
Psychoneuroimmunology wikipedia , lookup
Adoptive cell transfer wikipedia , lookup
Transcript
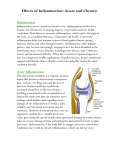
Inflammation Regulation Drug Traumeel IAH AC Inflammation Regulation Drug Traumeel © IAH 2007 In this course Traumeel is presented as a new class of medication used to treat inflammatory processes. In fact Traumeel is not a classical NSAID but an Inflammation Regulating Drug. Argumentation for that is the basis for this lecture. 1 Physiological processes • Most physiological processes in the human body are autoregulative • Respiration • Blood pressure • Heart rhythm • Defense system • …. • Auto-regulation is needed to adapt the organism’s reaction versus changing impulses out of the proper body and environment. © IAH 2007 2 Most physiological processes in the human body are steered over auto-regulating systems. Around an adaptable set point these systems will balance in a wave pattern by alternating action of agonists and antagonists. Most steering activities are induced over mediators like chemokines, hormones, interleukins, etc… Examples of these auto-regulating systems are the blood pressure, heart rhythm, defense activities, sleep-awake status, female cycle, muscle tonus, respiration rhythm, and many others. Autoregulation systems can be defined as biological systems equipped with inhibitory feedback systems such that a given change tends to be largely or completely counteracted. The forward output of such a system, will thus immediately trigger the corresponding inhibitory system so that overshoot of the process is prevented, and after the offending stimulus is removed, the system can return to baseline In the organism, trigger and feedback interactions are subtle, using micro or even nano-dosages of mediators. Diseases can be defined as caused by deregulations of these processes, important deviations of the set points or even completely changed set points that are not purposeful anymore. 2 Auto-regulating systems in the body • Have an adaptive set point • Have positive or negative feed back systems to keep control over the output • Use agonists and antagonists to steer • Are purposeful • Can deregulate • Can get rigid © IAH 2007 3 Auto-regulating systems in the body have some remarkable characteristics. They have an adaptive set point which means that the system will adapt to circumstances and influences in a regulative way. By the feed back system wave patterns of correction and adaptation are used to stay regulated as close as possible to the set point. The wave pattern is continuous and in no way the adaptation to a new circumstance is linear. To steer the adaptation agonists and antagonists are used alternating in overweight to ‘balance’ around the set point. The auto-regulation systems are purposeful, which means that the energy used to realize the objective is minimal for a maximal result. Auto-regulating systems can deregulate or even block due to external influences or false feed back information. Deregulated information streams will almost always lead to disease. 3 The defense system is an autoregulation system to defend the organism © IAH 2007 An important example of a changed auto-regulation system in the body’s defense is an inflammation. Inflammation is a cleansing process, mainly taking place at the level of the extracellular matrix, trying to get rid of causal foreign or body own toxins. To do this a mostly local mobilization of defense cells is created to attack and eliminate the toxins. 4 Inflammation is an auto-regulated mainly local manifestation of defense creating: • Pain • Swelling • Redness • Local temperature increase • Loss of tissue function © IAH 2007 5 The pain, swelling, redness, temperature increase and loss of the function of the affected tissue (Calor, Rubor, Dolor, Tumor, and function loss or function laesae) are only the expression and result of what is going on in the organism: a trial to adapt defense regulation to the intoxicated status. At the same time that the inflammatory mediators are secreted, the corresponding anti-inflammatory mediators will be secreted so that the inflammatory and anti-inflammatory effect will be balanced till homeostasis is once more reached after the disturbance is dealt with. 5 The inflammation cascade TH-0 antigen APC TH-1 TH-2 Cellular defense CD8+ Humoral defense BC MF © IAH 2007 EOP MC 6 Inflammation is a complex process of enhanced defense that over diverse trigger and regulation mechanisms is steered. Important in this is the fact that antigen coming into the body quickly will be detected by antigen presenting cells (APC). In the major histocompatibility complex class II (MHC-class II) of the APC characteristic proteins of the antigen will be presented to trigger unspecific and specific defense against this antigen. Pro-lymphocytes will become competent T helper cells due to the fact that they ‘blueprint’ the MHC information of the APC. In this way a TH-1 pathway is created that will take care of a stimulated cellular defense, mainly by activation of macrophages and cytotoxic cells (cT cells or CD8+ cells). Over this pathway damaged and by antigen intruded cells (e.g. viruses) will be eliminated. On the other hand there is the creation of a TH-2 pathway out of the same MHC-information that will create and enhance a humoral defense against the antigen. This is mainly done over B-cell activation, triggering of eosinophiles and mast cell degranulation. The last one will, over the release of histamine ameliorate defense transport and over the release of phospholipids lead to the formation of pro-inflammatory mediators like prostaglandins and leukotrines. 6 Conventional medicine inflammation approach • Inflammation is seen as disease and should be inhibited by all means • Conventional anti-inflammatory medication blocks off the regulation process that steers the cleansing activity of the defense system © IAH 2007 7 In conventional medicine inflammation is not seen as a cleansing or restoring process but as the characteristic of a disease. Therefore it should be inhibited by all means to regain health. The inflammation can be primary or secondary but the conventional approach remains the same: anti-inflammatory medications are used to block the cascade of the inflammation process. 7 Conventional medicine inflammation approach • By using all kind of suppressing medication that intervenes in the inflammatory process • Common NSAID’s • COX-II inhibitors • 5-LOX inhibitors • Corticoids • Antihistaminica • Mastcell stabilizers •… © IAH 2007 8 Most conventional medications used in inflammation have one characteristic in common: in one way or another they block of a pathway of the inflammation process. Blocked aspects can be an enzyme (e.g. cyclooxygenase, 5lipooxygenase,…) or a receptor (H1-blockers, mast cell blockers in hay fever,…). Most common is the use of the broad range of NSAIDs and the more specific class of the COX-II inhibitors. Beside a suppression of the inflammation symptoms they all will intervene in the regulation systems of the inflammation cascade and therefore deregulate the natural process of defense that was meant to cleanse the body. From homotoxicological point of view conventional anti-inflammatory medications are pushing the organism from a fight to a deposition reaction which means that causal homotoxins will ‘condensate’ in the extracellular matrix. 8 Main side effects of conventional NSAID’s • Gastro-intestinal disorders • Blood platelet aggregation inhibited (anti-tromboxane effect, risks on bleedings) • Renal impairment (can lead to sodium and water retention) © IAH 2007 9 Blocking the COX pathway with NSAIDs will be compensated by the organism by a higher LOX pathway (side effects: bronchial spasm due to higher leukotrines) and problems with higher thrombotic rates due to an increase in tromboxanes (COX-II problematic). As the organism tries by all means (all inflammatory pathways and the metalloproteinases) to eliminate the causal intoxication, any suppression of a pathway will be compensated by an increased other proinflammatory pathway like communicating vessels will show increased levels if one of them has been suppressed. The consequences of blocked inflammatory pathways and compensation by other inflammatory pathways leads to gastro-intestional disorders and blood platelet aggregation disturbances. Some of the molecules used in conventional treatment of inflammation are nephrotoxic and can lead to renal impairment. 9 Contraindications of conventional NSAID’s • Severe heart failure • Gastric and duodenal ulceration • If hypersensitivity is present against ASA or any other NSAID molecule • In severe renal and hepatic impairment • COX-II should be used with care in cardiac failure © IAH 2007 10 Due to direct or side effects classical NSAIDs are contraindicated in severe heart failure and gastric and duodenal ulceration. Some people are hypersensitive, intolerant or allergic to acetyl salicylic acid or other anti-inflammatory components used in NSAIDs and therefore should not use these type of medications. Due to neprotoxicity and hepatoyicity NSAIDs are contraindicated in severe renal and hepatic impairment. Especially the class of COX-II inhibitors should be used with care in cardiac failure. 10 Homotoxicological definition of illness • Diseases are the expression of biologically purposeful defense mechanisms against endogenic and exogenic homotoxins, or the expression of the organism’s effort to compensate for toxic damages it has sustained. © IAH 2007 11 From a homotoxicological point of view, illness is caused by the body’s reaction to the presence of disruptive homotoxins. What we recognize as the clinical symptoms of illness is what surfaces after the defense system has reacted to the threat. This means that illness is not the presence of symptoms as such, since these should only be seen as proof of ongoing defense activity. As long as clinical symptoms are only viewed as a threat to the quality of life of the patient and the entire treatment is geared to the removal of these symptoms, the results will be superficial and we are actually mortgaging the patient’s long-term health. A biotherapeutic treatment takes into account the causative homotoxins and, by stimulating the body’s own defense system, will affect the actual causes of the illness. Biotherapy is always a regulation therapy, and never a suppression therapy. Purposeful: This term is extremely important in the homotoxicological definition of disease. It means that the reaction of the defense system will be in proportion to the needs to reach the target. This encompasses every regulation aspect homotoxicology is referring to. Mobilization of defense will be at the level that is needed to reach the target which in most cases is the elimination of the homotoxin and his negative interactive activity with the cell and his environment and to restore homeostasis. The regulation of the level of activity is done over a complex mechanism of autoregulating systems, interacting one another and this over many different mediators and feedback systems. Most reactions of the defense system are purposeful, but inappropriate reactions (unpurposeful) can occur and create diseases on their own. Auto-immune diseases for example are an inappropriate reaction of the defense system. The immune system is attacking body own tissues which under normal conditions would be tolerated instead of attacked. The same is true for allergic reactions such as hay fever. The reaction of the defense system is not in relationship to the danger of the aggressor (the dust or the pollen) and therefore not purposeful. 11 Homotoxicological definition of inflammation • Inflammation is the expression of biologically purposeful defense mechanisms against endogenic and exogenic homotoxins © IAH 2007 12 In the same way we could define inflammation as an expression of biologically purposeful defense. The inflammation process is meant to eliminate toxic burden or damage and restore a healthy situation by local mobilization of defense cells. 12 Inflammation in antihomotoxic medicine • Inflammation is a cleansing process by which homotoxins are eliminated, mostly at the level of the extracellular space • Inflammation is steered by auto-regulating systems • The steering is mainly done over all kind of mediators • Cytokines like interferons (e.g. IFN-γ,…), interleukins (e.g. IL-2, IL-8,… and chemokines (TNF-α, TGF-β,…) • Hormones (e.g. DHEA, cortisol,…) • antihomotoxic medications intervene modulating in the regulation process © IAH 2007 13 So, to summarize, we can state that inflammation is a cleansing and restoring process that is steered by auto-regulating systems. Steering is done over the release of all kind of mediators like cytokines and hormones. With antihomotoxic medications we intervene modulating in these steering processes by stimulation or inhibition of the secretion of mediators by defense active cells. 13 Disease Evolution Table: Inflammation • Inflammation is the second phase • • on the table Left of the Regulation/Compensation Division Where the defense system reacts with regulation to intoxication Inflammation Phases © IAH 2007 14 In the disease evolution table (DET) inflammation is the second phase after enhanced excretion. In fact by regulating defense against the present homotoxins the extracellular terrain of the patient is cleansed so that cell function is not endangered anymore. All acute inflammations in different tissues and organs are classified in this second phase. Suppression of the inflammation process will push the patient to the next phase which is the deposition phase. In this third phase the homotoxins are no longer eliminated but get stored in the extracellular matrix where on long term they will obstruct or deregulate a proper cell function. 14 The Immunological Bystander Reaction (IMBR) and antihomotoxic medications (Prof. H. Heine) Antihomotoxic preparation The D refers to different potentizations of substances. D4 - D8 is a selection from a range of D1 - D14 oral s. c. nasal i. v. aerosol i. m. Absorption Macrophage Processing Mediators which activate ground regulation Major histocompatibility complex (MHC) Differentiation of the T-cells into regulating Th3 with motif Motif formation (5-15 amino acids) Homing Similarity recognition (Simile principle) regulatory lymphocytes (Th3) e.g. inflamed joint T-cell (prolymphocyte) Immunoglobulinproducing B lymphocytes Lymph node Organotropism Histotropism TGF-β IL-4 IL-10 Suppresion of the matching Th1, Th2 Inflammation-promoting lymphocytes (Th1, Th2) Clone formation in lymph nodes © IAH 2007 15 The immunological bystander reaction, a principle in modern immunology, was used by Prof. Hartmut Heine to postulate the working mechanism of some low concentrated organic components in antihomotoxic medications. Prof. Hartmut Heine was a histologist and at the end of 1997 he published his hypothesis of the “Immunological Bystander Reaction pathway”, a pharmacodynamic model (proven in vitro) for organic substances in the range of D1 to D14. Heine’s model is very important. Minute molecular concentrations of organic components, like in the formula of Traumeel, stimulate a TH-3 mediated immunological reaction. The figure above explains Heine’s model. Where an antihomotoxic agent with low potentized proteins is introduced into the GRS (Ground Regulation System) antigen presenting cells and dendritic cells will eliminate it over phagocytosis. Characteristic proteins are transported back to the macrophage’s surface in the form of short amino acid chains. These presentation is antigen characteristic and specific. It is presented as a ‘motive’ in the MHC class II on the cell surface (MHC – Major Histocompatibility Complex). 15 The Immunological Bystander Reaction (IMBR) and antihomotoxic medications (Prof. H. Heine) Antihomotoxic preparation The D refers to different potentizations of substances. D4 - D8 is a selection from a range of D1 - D14 oral s. c. nasal i. v. aerosol i. m. Absorption Macrophage Processing Mediators which activate ground regulation Major histocompatibility complex (MHC) Differentiation of the T-cells into regulating Th3 with motif Motif formation (5-15 amino acids) Homing Similarity recognition (Simile principle) regulatory lymphocytes (Th3) e.g. inflamed joint T-cell (prolymphocyte) Immunoglobulinproducing B lymphocytes Lymph node Organotropism Histotropism TGF-β IL-4 IL-10 Suppresion of the matching Th1, Th2 Inflammation-promoting lymphocytes (Th1, Th2) Clone formation in lymph nodes © IAH 2007 16 These motifs or patterns are recognized by passing naïve T-lymphocytes, which over their receptors will interact with them. So, over the TCRs (T cell receptors) of their own and the motif presented by the APC there is an interaction. This interaction is the signal for them to become TH-3 cells (regulating lymphocytes). The new TH-3 cells will be transported to the closest lymph ganglion (homing) where they will be identically multiplied (cloning). The activated TH-3 cells search for inflammation promoting lymphocytes (TH-1, TH-2) in the inflammation area, which motives are dependent on the foreign substances that triggered the inflammation. The TH3-cell will look for lymphocytes with a similar motive. As soon as the similarity is confirmed, the TH-3 cells immediately start with the synthesis of highly active TGF-ß (Transforming Growth Factor ß), which will decrease the activity of the TH-1 and TH-2 lymphocytes. The inhibition of the TH1 and TH-2 accuracy will result in an inhibition of the inflammation stimulation by these lymphocytes, which will result in less inflammation symptoms and activity. In one sentence we could state that Traumeel stimulates the creation of specific TH-3 cells that by the release of TGF-β will inhibit the TH-1 and TH-2 activity. 16 3 action pathways known NF-κβ Traumeel IL-1 TGF-β TNF-α © IAH 2007 17 Traumeel has inflammation regulating capacities and therefore is called an inflammation regulating drug (IRD). 3 main pharmacokinetic pathways are known out of basic research. Components of Traumeel will influence the nuclear transcription factor kappa beta (NF-κβ) in this way that lesser pro-inflammatory genes are triggered to stimulate the secretion of pro-inflammatory mediators out of the cell. Another action of Traumeel is the inhibition of IL-1 and TNF- α, two strong pro-inflammatory mediators that are down regulated. The third known pharmacokinetic action is by stimulating the formation of specific Treg cells (TH-3 lymphocytes known as a variant of CD4+ cells) that by the release of transforming growth factor beta (TGF-β) will inhibit pro-inflammatory Th-1 and TH-2 cells. 17 3 action pathways known Traumeel TGF-β © IAH 2007 18 Prof. H. Heine postulated that one of the main activities of Traumeel lies in its ability to stimulate the production of specific TH-3 (T-regulating cells) that by the release of TGF-β will inhibit the activity of proinflammatory TH-1 and TH-2 cells[1]. Basic research to which forms the foundation to this postulation was the 1995 research of Prof. Weiner at the University of Boston. He found out that specific proteins in high dosages will induce the production of pro-inflammatory CD4+ T lymphocytes (TH-1 and TH-2 cells) whereas micro doses of this same protein will induce the production of anti-inflammatory CD4+ T lymphocytes (Treg cells). This phenomenon is called ‘oral tolerance’ in immunology and refers to the fact that the organism is programmed to be tolerant if exposed to minor quantities of a foreign protein (if not the body would react in defense to everything it meets, in toxic quantities or not). Heine did basic research on TGF-β production of small dosages of different proteins (plants and organ extracts) in full blood cultures and his approach seemed to be confirmed for many of them. Although not all proteins seem to trigger a TGF-β production, the main components of Traumeel do so. TGF-β has two main effects that are extremely interesting to understand the therapeutic results we have with Traumeel. As mentioned above, TGF-β will inhibit pro-inflammatory T cells with a similar motive on their TCR as the Treg cell has. Above that TGF-β has a strong stimulating effect on fibroblasts. Fibroblasts generate the proteoglycan (PG) and glycosaminoglycan (GAG)-matrix at the level of the extracellular environment. Fibroblasts also repair damaged collagen. During the shock phase of an inflammation (acid phase) the PG and GAG structure is degraded but in the anti-shock phase the structure is repaired by the fibroblasts. TGF-β will directly stimulate the fibroblast’s action and create a new matrix in a much shorter time. As the matrix is the basic structure for wound healing this explains the value of Traumeel in wound healing and traumata of different origin. As the matrix is also a biophysical filter that will select the transmission of substances traveling from the capillaries to the cells and vice versa the quality of the life of the cell depends directly on the quality and purity of that matrix. More than one reason enough to repair it faster when damaged by inflammation and to keep it as pure as possible by accurate and purposeful defense. [1] Heine H, Schmolz M. Immunologische Beistandsreaktion durch pflanzliche Extrakte in Antihomotoxischen Präparaten. Biologische Medizin. 1998, 27 (1), 12-14. 18 3 action pathways known Traumeel IL-1 TNF-α © IAH 2007 19 Other recent basic research[1] showed the inhibiting effect of Traumeel on IL-1β and TNF-α. Both mediators are cortisol antagonists. In fact they are TH-1 mediated inflammation promoters as cortisol is the negative feedback mechanism to IL-1 and TNF-α. In the balance of agonists and antagonists a IL-1β and TNF-α inhibition will favor the cortisol effect which is an anti-inflammatory effect. Above that new research shows that cortisol in fact has some of its peripheral antiinflammatory effect via the secretion of TGF-β . It is clear that due to this effect we see a strong reduction of the clinical symptoms in inflamed patients using Traumeel. [1] Porozov S, Cahalon L Weiser M, Branski D, Lider O, Oberbaum M., Inhibition of IL-1β and TNF alpha Secretion from Resting and Activated Human Immunocytes by Homeopathic Medication Traumeel S. Clinical & Developmental Immunology. 2004 ;11(2): 143-149. 19 3 action pathways known NF-κβ Traumeel © IAH 2007 20 A particular role in the inflammation process is filled in by NF-κβ or the nuclear transcription factor kappa beta. Free radicals, bacterial products and cytokines bind to receptors on the cell wall and then will through a series of intermediate messengers cause the release of NF κ B. NF-κβ will migrate to the cell nucleus where it triggers the transcription of inflammatory mediators, such as IL 1 and TNFα. Components of the Traumeel formula (of the asteracea family) contain helenanin which is a sesquiterpene lactone that strongly inhibits NF-κβ. So, we can postulate that Traumeel directly also inhibits NF-κβ and over this inhibits the release of pro-inflammatory cytokines by the cell. This postulation fits with results from above mentioned basic research on inhibiting effect of Traumeel on IL-1 and TNF-α. Both mediators are strong inducers of NF-κβ migration to the nucleus. The inhibiting effect of IL-1 and TNFα has a synergic effect with the direct inhibition of NF-κβ by the helenanin containing Traumeel components. 20 Traumeel stimulates TGF-β secretion • Traumeel stimulates the formation of specific T-regulator cells (TH-3: CD4+ inflammation inhibiting cells) that by the secretion of TGF-β will inhibit the TH-1 and TH-2 cells (CD4+ proinflammatory T cells) • TGF-β inhibits at the beginning of an inflammation cascade (top: pro-inflammatory T-cells) • TGF-β stimulates the activity of fibroblasts (matrix generation: better tissue and wound healing) © IAH 2007 21 As in the inflammation cascade Treg cells intervene with TGF-β to down regulate pro-inflammatory CD4+cells, Traumeel components will regulate inflammation at the top of the cascade. The higher in a chain reaction we intervene regulating, the more parameters will be involved in that regulation. 21 The inflammation cascade TH-0 antigen APC TH-1 TH-2 Cellular defense CD8+ Humoral defense BC MF © IAH 2007 EOP MC 22 Inflammation is a complex process of enhanced defense that over diverse trigger and regulation mechanisms is steered. Important in this is the fact that antigen coming into the body quickly will be detected by antigen presenting cells (APC). In the major histocompatibility complex class II (MHC-class II) of the APC characteristic proteins of the antigen will be presented to trigger unspecific and specific defense against this antigen. Pro-lymphocytes will become competent T helper cells due to the fact that they ‘blueprint’ the MHC information of the APC. In this way a TH-1 pathway is created that will take care of a stimulated cellular defense, mainly by activation of macrophages and cytotoxic cells (cT cells or CD8+ cells). Over this pathway damaged and by antigen intruded cells (e.g. viruses) will be eliminated. On the other hand there is the creation of a TH-2 pathway out of the same MHC-information that will create and enhance a humoral defense against the antigen. This is mainly done over B-cell activation, triggering of eosinophiles and mast cell degranulation. The last one will, over the release of histamine ameliorate defense transport and over the release of phospholipids lead to the formation of pro-inflammatory mediators like prostaglandins and leukotrines. 22 The inflammation cascade TH-0 antigen APC TH-1 TGF-β Cellular defense CD8+ TGF-β Humoral defense TH-3 BC MF © IAH 2007 TH-2 EOP MC 23 The TH-3 down regulation over TGF-β is seen on the picture above. TGF-β inhibits TH-1 and TH-2 activity and is an important regulator in keeping the inflammation process within reasonable limits. As shown on the picture this down regulation is at the top of the inflammation cascade and not at the bottom as is the fact in conventional medications like NSAIDs (prostaglandin-synthesisinhibitors). 23 TH0 DH EA Co rti s ol TH3 TH2 TH1 IL-2 IL-4, 13 IFN gamma TGF-beta IL-5 IL-10 TNF Inflammation © IAH 2007 Inhibition Allergy 24 Pregnenolone and subsequently DHEA are called the mother of hormones by researchers because it is used by the body to manufacture many other hormones, including our sex hormones that are necessary for many body functions (e.g. estrogen, testosterone, progesterone, cortisol,…) They are responsible for the maintenance of many body functions such as fat and mineral metabolism, controlling stress, maintaining male and female characteristics and others. The body produces DHEA and then converts it on demand to these other hormones. DHEA stimulates cellular immunity or skewing of TH-1 mediated reaction in the balance where as cortisol inhibits TH-1 reactions but will stimulate on long term TH-2 mediated reactions. We can state that a long term secretion of cortisol (e.g. stress) will induce a skewing at the TH-2 side of the balance inducing allergies, some auto-immune diseases and even cancer. Some auto-immune diseases are TH-1 driven (e.g. morbus Crohn) As TH-3 regulator cells, over the release of TGF-β, inhibit both sides of the balance (down regulation) it does not directly intervene in the dysbalance but in the intensity of both the TH-1 or TH-2 expression. We know from research1 that Traumeel inhibits the sectretion of IL-1β and TNFα. These two mediators inhibit cortisol production. That is why Traumeel has such an infammation down regulating effect, amongst others. 1. Porozov S, Cahalon L Weiser M, Branski D, Lider O, Oberbaum M., Inhibition of IL-1β and TNF alpha Secretion from Resting and Activated Human Immunocytes by Homeopathic Medication Traumeel. Clinical & Developmental Immunology. 2004 ;11(2): 143-149. 24 Inhibiting Interleukines and other mediators activating NK-cells dendritic cells IL-12 TH-2 F-β TG TH-3 Mast cells TGF IL-4 -β Eosino- IL-10 phile TH-1 TH-2 IFN-γ IL-2 IFN-γ IL-2 IFN-γ IFN -γ/ TN Fβ IL-12 IL-4 IL-5 IL-3 IL-4 IL-6 IL-13 IL-10 B-cell B-cell cT-cell IgG2a activation macrophage activation © IAH 2007 Mast cell Other Ig classes growth 25 A large number of mediators regulate an immune response. Although both sides of the TH1/TH2 balance induce different actions they both are able to ‘control’ and inhibit reciprocally their own actions. A TH1 mediated pathway will inhibit over the release of interferon gamma the TH2 pathway and the other way around the TH2 cell can over the release of interleukin 10 inhibit the TH1 pathway. Above TH1 and TH2 stands the regulator cell (TH3 or Treg cell) that over the release of transforming growth factor beta can inhibit both the TH1 and TH2 pathway. In function of the cellular or humoral defense different immunocytes are activated. In both pathways the activity of the ending cell in the cascade influences the input of the pathway. Macrophages stimulate TH1 activity over the release of IL-12 but are activated themselves by the release of IFN-γ and TNF-β, both released by the TH1 cell. In this way a loop is created. A similar loop is seen in the TH2 pathway. Mast cells induce TH2 activity that over the release of Interleukin 3, 4 and 10 will activate the mast cell. To conclude we can state that both the TH1 and TH2 pathways, over positive feedback, stimulate their own loop which is only inhibited by the reciprocal inhibition between TH1 and TH2 and the supervising regulating effect of the Treg cells. 25 © IAH 2007 26 In an inflammation we see TH-1 and TH-2 pathways alternating around a set point of balance. Desoxicortisol will stimulate a TH-1 state there as cortisol will induce a TH-2 state. An overweight of one of the two will cause a prolonged characteristic status of cellular or humoral defense with all the consequences mentioned already before. Keeping the wave pattern moving until a harmonious set point is reached or getting as close as possible to that is one of the targets of immunomodulation. The picture always explains the danger of the prolonged TH-1 blocking use of corticoids in conventional therapy as this medication will push and keep the patient into a TH-2 mediated defense with all his consequences. 26 Traumeel S inhibits pro-inflammatory cytokines • Traumeel is proven to have strong inhibiting effects on IL-1β, TNF-α and IL-8(1) • IL-1β and TNF-α are pro-inflammatory cytokines that have a strong antagonistic effect on cortisol • By inhibiting IL-1β and TNF-α the effect of the body’s own effect of cortisol becomes more pronounced. 1) Porozov S, Cahalon L Weiser M, Branski D, Lider O, Oberbaum M., Inhibition of IL-1β and TNF alpha Secretion from Resting and Activated Human Immunocytes by Homeopathic Medication Traumeel S. Clinical & Developmental Immunology. 2004 ;11(2): 143-149. © IAH 2007 27 27 Traumeel S inhibits nuclear transcription factors • NF-κβ is a complex of nuclear transcription factors that under the impulse of antigens migrates to the cell nucleus and triggers specific genes to stimulate the cell to release pro-inflammatory cytokines (like TNF-alpha) • Asteracea in Traumeel S inhibits NF-κβ migration © IAH 2007 28 28 Traumeel indication • Traumeel is suitable for the treatment of • primary and secondary inflammations, especially of the musculoskeletal system (arthritis, inflammatory aspect in reactivated arthrosis, PHS, tendonitis,…) • traumata of all kind • sport injuries (bruises, distortions, sprains, micro-lesions after • performance,…) damaged soft tissues © IAH 2007 29 Due to his inflammation regulating capacities Traumeel can be used for the treatment of most primary and secondary inflammations. Although this broad indication area Traumeel is mainly used for inflammations of the locomotorial system. It is a great medication in the treatment of sport injuries as most of these disorders will go together with inflammation. Above that, due to the micro and nano dosages of the components, doping risks are excluded. 29 Pharmacokinetics synthesis • TGF-β inhibits both TH-1 and TH-2 mediated pro-inflammatory pathways • IL-1 and TNF-α are released due to inflammatory impulses • Antigens will trigger on cell receptors the decomposition of NF-κβ and stimulate the release of pro-inflammatory cytokines, due to specific gene stimulation • Hypothesis: TGF-β is responsible for IL-1, TNF-α and NF-κβ inhibition in the inflammatory cascade. © IAH 2007 30 30 Traumeel formula • Achillea millefolium • Aconitum napellus • Arnica montana • Atropa belladonna • Bellis perennis • Calendula officinalis • Chamomilla recutita • Echinacea angustifolia © IAH 2007 • Echinacea purpurea • Hamamelis virginiana • Hepar sulfuris • Hypericum perforatum • Mercurius solubilis Hahnemanni • Symphytum officinalis 31 The formula of Traumeel consists of 14 components in very subtle dosages, covering the main symptoms of an inflammation. 31 Traumeel formula Antisuppurative action Increase of tone and stabilization of vasal permeability, blood stagnation, venous stasis Echinacea ang and purp., Mercurius sol. Hahn., Hepar sulfuris Aconitum, Hamamelis, Millefolium, Bellis, Belladonna, Arnica Inflammation Stimulation of circulation Calendula, Arnica, Echinacea purp., Symphytum Analgesic Aconitum, Arnica, Chamomilla, Hypericum © IAH 2007 32 As seen on this slide there are mainly 4 clinical effects created after the use of Traumeel. A group of components will intervene at the level of the vasal permeability and tonus and the venous stasis. Other components create an antisuppurative effect. The third group of components have an analgesic effect and the last group will stimulate the circulation. All together, beside the inflammation regulating effect, we see a fast and positive effect in decreasing the clinical image of inflammation. 32 Traumeel galenic forms • Injections: 1 to 3 times 1 ampule a day in i.m, i.c., s.c., or periarticular • Drops: 3 times 10 drops a day • Tablets: 3 times 1 tablet a day (by preference to be solved in the mouth) • Ointment: local application 2 times a day, eventually under bandage • Gel*: local application 2 times a day • Drinking ampules*: one ampule a day * in function of the country © IAH 2007 33 Worldwide 6 galenic forms are available. The dosage and frequency is mentioned on the slide per galenic form. Not all forms might be available in every country worldwide but if available the dosage and frequency remains the same. 33 Traumeel: synthesis • No NSAID but Inflammation Regulating Drug (IRD) • In inflammations of the locomotorial system and sport injuries • Arthritis, tendonitis,… • Distortions, bruises,… • Traumeel has been shown in vitro to: • Modulate pro-inflammatory Th-1 and Th-2 cells (TGF-β)1 • Modulate IL-1β, TNF-α and IL-82 • Safe and well tolerated • Very effective 1. Heine,H.: Immunological Bystander Reaction 2. Porozov S, Cahalon L, Weiser M, Branski D, Lider O, Oberbaum M. Inhibition of IL-1beta and TNF-alpha Secretion from Resting and Activated Human Immunocytes by the Homeopathic Medication Traumeel S. Clinical and Develomental Immunology. 2004;11(2):143-149. © IAH 2007 34 To synthesize we can state that Traumeel is an IRD used in inflammations and sport injuries. Traumeel has regulating effects over a modulation of inflammation mediators. It is proven to be a safe and well tolerated medication with a high effectiveness in the mentioned indications. 34