* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Chapter 5
Molecular Hamiltonian wikipedia , lookup
Particle in a box wikipedia , lookup
Franck–Condon principle wikipedia , lookup
Hydrogen atom wikipedia , lookup
Rutherford backscattering spectrometry wikipedia , lookup
Wave–particle duality wikipedia , lookup
Auger electron spectroscopy wikipedia , lookup
Molecular orbital wikipedia , lookup
Atomic theory wikipedia , lookup
X-ray fluorescence wikipedia , lookup
Theoretical and experimental justification for the Schrödinger equation wikipedia , lookup
Tight binding wikipedia , lookup
Electron scattering wikipedia , lookup
X-ray photoelectron spectroscopy wikipedia , lookup
Electron-beam lithography wikipedia , lookup
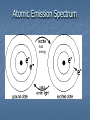
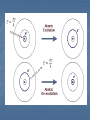
CHAPTER 5 ELECTRON CONFIGURATION ANALOGY OF THE ATOM UNITED STATES NEW JERSEY MIDDLESEX COUNTY EDISON Township YOUR HOUSE ATOM ENERGY LEVEL SUBLEVEL ORBITAL LOCATION OF ELECTRON 1.Principle Energy Levels: (n = 1,2,3….7) a.k.a shells Different values of n = different energy levels & different electron energies Maximum number of e- each level holds is found by: 2n2 EX: 1st energy level (n=1) 2n2 = 2(1)2 = 2 electrons in 1st energy level Increasing energy level n= 1 2 Max # 2 8 of e-s Increasing distance from nucleus 3 4 18 32 2.Sublevels: s (spherical), p (dumbbell), d, f Cloud shapes The # of energy sublevels is the same as the energy level # Energy Level (n) # of Sublevels Type of Sublevel n=1 1 1s n=2 2 2s2p n=3 3 3s3p3d n=4 4 4s4p4d4f 3.Orbitals: Each sublevel has a different # of orbitals which means a different # of electrons The # of orbitals in an energy level is found by: n2 EX: 3rd energy level (n=3) 32 = 9 orbitals This makes sense because: 3rd energy level would have 3 sublevels; s sublevel with 1 orbital, p sublevel with 3 orbitals, and d sublevel with 5 orbitals. SO……1 + 3 + 5 = 9, so the formula n2 works! Sublevel(s) # of orbitals n2 s 1 Maximum # of e-s 2n2 2 s,p 4 8 s,p,d 9 18 s,p,d,f 16 32 PRACTICE Principle Energy Level 5 2p Number of Electrons in Sublevel Type of Electron Sublevel Orbitals and Electron Capacity of the First Four Principle Energy Levels # of Maximum Principle # of Type of orbitals # of energy orbitals sublevel per level electrons level (n) per type (n2) (2n2) 1 s 1 1 2 s 1 2 4 8 p 3 s 1 3 p 3 9 18 d 5 s 1 p 3 4 16 32 d 5 f 7 HOW TO DETERMINE ELECTRON CONFIGURATIONS How electrons are arranged around the nuclei of atoms 3 RULES: Aufbau Pauli Exclusion Principle Hund’s Rule Aufbau Principle: Electrons enter orbitals of lowest energy level 1st Start at the beginning of each arrow, and then follow it all of the way to the end. 1s 2s 2p 3s 3p 4s 3d 4p 5s 4d 5p 6s 4f 5d 6p 7s 5f 6d 7p 2. Pauli Exclusion Principle: Orbitals can hold only 2 electrons Each electron in the orbital has an opposite spin 1s2 3. Hund’s Rule: One electron enters each orbital until all orbitals contain one electron with parallel spins. 1s2 1s2 2s2 2s2 2p3 2p4 Bohr ModelLine Spectra Explained Electrons can occupy specific energy levels Excited atoms can emit light Each orbital they are in has specific energy. Atomic Emission Spectrum Spectrometer: Breaks up what we see as continuous light into individual bands of light. The individual bands of light represent the exact frequency of light be given off. This corresponds to the quantum of energy that is released when an electron goes from an excited state to the ground state. Electrons move from ground state to an excited state and back again. The bands of light are called: ATOMIC EMISSION SPECTRUM This spectrum is unique to every element. Atomic Emission Spectrum Add energy Different elements are used to give fireworks their color! To learn more about the science of fireworks see: http://www.pbs.org/wgbh/nova/kaboom/ Electromagnetic Spectrum How Does the Atomic Emission Spectrum Correspond to Electron Configuration? 1. Electrons get excited when we introduce ENERGY (fire, electricity, light) 2. Electrons jump from ground state to an excited state 3. The amount of energy needed to do this is a QUANTUM of energy QUANTUM: is the amount of energy needed to move an electron from one energy level to the next. 4. Electrons go back to the ground state and give off this energy in the form of light (PHOTONS) PHOTON: a quantum of light; a discrete amount of energy that behaves as a particle. E= h c / l E= Energy h= Planck’s constant c= speed of light l=wavelength