* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Combinatorial signals from the neural tube, floor plate and
Survey
Document related concepts
Transcript
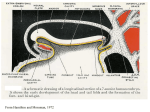
Development 121, 651-660 (1995) Printed in Great Britain © The Company of Biologists Limited 1995 651 Combinatorial signals from the neural tube, floor plate and notochord induce myogenic bHLH gene expression in the somite Andrea E. Münsterberg and Andrew B. Lassar Department of Biological Chemistry and Molecular Pharmacology, Harvard Medical School, 240 Longwood Avenue, Boston, MA 02115, USA SUMMARY The neural tube, floor plate and notochord are axial tissues in the vertebrate embryo which have been demonstrated to play a role in somite morphogenesis. Using in vitro coculture of tissue explants, we have monitored inductive interactions of these axial tissues with the adjacent somitic mesoderm in chick embryos. We have found that signals from the neural tube and floor plate/notochord are necessary for expression of the myogenic bHLH regulators MyoD, Myf5 and myogenin in the somite. Eventually somitic expression of the myogenic bHLH genes is maintained in the absence of the axial tissues. In organ culture, at early developmental stages (HH 11−), induction of myogenesis in the three most recently formed somites can be mediated by the neural tube together with the floor plate/notochord, while in more rostral somites (stages IVIX) the neural tube without the floor plate/notochord is sufficient. By recombining somites and neural tubes from different axial levels of the embryo, we have found that a second signal is necessary to promote competence of the somite to respond to inducing signals from the neural tube. Thus, we propose that at least two signals from axial tissues work in combination to induce myogenic bHLH gene expression; one signal derives from the floor plate/notochord and the other signal derives from regions of the neural tube other than the floor plate. INTRODUCTION opmental stages somitic cells are committed to particular cell fates and differentiate irrespective of their location within the embryo (Aoyama and Asamoto, 1988; Christ et al., 1992; Ordahl and LeDouarin, 1992). Efforts to identify the source of the cues that dictate somitic cell fate have focused attention on the axial tissues of the vertebrate embryo: the developing neural tube and the underlying notochord. It was noted that somites that form in the absence of a neural tube do not progress beyond a hollow epithelial sphere morphology reminiscent of immature somites, and therefore seem to be arrested in their maturation (Packard and Jacobson, 1976). Recently, it was demonstrated that excision of the neural tube and notochord from early chick embryos results in the striking absence of axial skeletal muscle (Christ et al., 1992; Rong et al., 1992). In vitro experiments have provided evidence that the neural tube directly promotes skeletal muscle differentiation (Avery et al., 1956; Vivarelli and Cossu, 1986; Kenny-Mobbs and Thorogood, 1987; Rong et al., 1992; Buffinger and Stockdale, 1994; Stern and Hauschka, 1994). In these assays, developmentally immature somites were explanted and cultured under various conditions; muscle differentiation markers (i.e. myoblast fusion or myosin heavy chain synthesis) were not activated unless the somites were co-cultivated with cells from the adjacent neural tube. In contrast, developmentally more mature somites could differentiate into muscle when explanted and cultured in the absence of neural tube. The initiation of the myogenic program is thought to be con- In all vertebrate embryos, the paraxial mesoderm gives rise to transient structures called somites. The somites are repeated units which are progressively generated during development along the anteroposterior axis on either side of the neural tube. Somite morphogenesis is outlined in Fig. 1. Initially, somites form as epithelial spheres from the unsegmented paraxial mesoderm. [Throughout this paper, we refer to the most recently formed somite as somite I, the next youngest somite as somite II, etc., (as proposed in Ordahl, 1993).] As development proceeds, a columnar epithelial sheet develops in the dorsal somite (dermomyotome), while cells in the ventral somite loose their epithelial morphology and give rise to a loosely connected mesenchyme (sclerotome). The sclerotome gives rise to precursor cells of the ribs, vertebrae and intervertebral discs (discussed in Christ and Wilting, 1992). Subsequently, cells of the dermomyotome that lie adjacent to the neural tube form an intermediate sheet of cells in the middle of the somite (myotome) that gives rise to axial skeletal muscle (i.e. vertebral and back muscle). Cells at the lateral edge of the dermomyotome migrate to give rise to the body wall/limb musculature (reviewed in Wachtler and Christ, 1992). Cells from the dorsalmost epithelial sheet (dermatome) give rise to dermis. At an early developmental stage, cell fate within the somite is plastic and responsive to extrinsic cues, while at later devel- Key words: neural tube, floor plate, notochord, myogenic bHLH, somitogenesis 652 A. E. Münsterberg and A. B. Lassar trolled by a set of basic-Helix-Loop-Helix (bHLH) transcription factors, which include MyoD, myogenin, Myf-5 and MRF-4 (reviewed in Buckingham, 1992; Emerson, 1993; Sassoon, 1993; Weintraub, 1993; Lassar and Münsterberg, 1994; Olson and Klein, 1994). Gene knockout by homologous recombination in mice has demonstrated that either MyoD or Myf-5 is necessary for the determination or survival of myogenic precursor cells and that myogenin is necessary to execute the differentiation program (Hasty et al., 1993; Nabeshima et al., 1993; Rudnicki et al., 1993). Detailed in situ hybridization studies have shown that the myogenic bHLH genes are expressed early during embryogenesis (Hopwood et al., 1989a; Sassoon et al., 1989; Bober et al., 1991; Charles de la Brousse and Emerson, 1990; Hinterberger et al., 1991; Ott et al., 1991; Pownall and Emerson, 1992) and, thus, they provide molecular markers for myogenic precursor cells in vivo. In different species, the order in which the myogenic bHLH factors are expressed differs; however, in all species examined, at least one member of this gene family is expressed in the early somite prior to its morphological segregation into dermatome, myotome and sclerotome (discussed in Buckingham, 1992). Interestingly, in both mouse and quail, myogenic bHLH transcripts first accumulate in the medial portion of the somite adjacent to the neural tube (Ott et al., 1991; Pownall and Emerson, 1992, Barth and Ivarie, 1994). In this report, we focus on the inductive interactions that are necessary to activate high level expression of the myogenic bHLH genes and induce skeletal muscle differentiation in somites. To study these interactions, we have dissected and cultured embryonic somites in the absence or presence of various axial tissues (neural tube, floor plate and notochord) and monitored the expression of skeletal musclespecific genes by RT-PCR. This approach has revealed that myogenesis of epithelial XI somites (stages I-III), isolated from stage 11− or younger chick embryos, requires interaction with both neural tube and floor plate in vitro. In contrast, myogenesis of more rostral somites (stages IV-IX), from V similar stage embryos, requires interaction only with the neural tube in the absence of floor plate. This finding suggests that two III II signals are necessary for somite myogenesis I in vitro. We propose a model in which interaction with the floor plate/notochord psm mediates the competence of the somite to respond to a muscle-promoting signal from the neural tube. MATERIALS AND METHODS Chick embryo dissections and culture of tissues Fertilized chick eggs (Spafas) were incubated at 38.5°C in a humidified incubator (Petersime). Embryos were staged according to Hamburger and Hamilton, 1951, collected in silicon (Dow Corning)-coated Petri dishes containing Tyrodes buffer (10× stock: NaCl 80 g/l, KCl 2 g/l, CaCl2 2 g/l NaH2PO4 0.5 g/l, MgCl2 2 g/l, Glucose 10 g/l) and pinned down with their ventral side facing up using insect pins. The endodermal epithelium was removed from the region to be dissected, and the following tissues were explanted: somites alone; somites with the adjacent neural tube, floor plate and notochord; somites with the adjacent neural tube excluding floor plate; or somites with floor plate and notochord (see figure legends for details and stages of embryos). Following dissection with micro feather scalpels (Oasis, CA) the tissues were transferred into Tyrodes buffer containing Dispase (Boehringer Mannheim, 1 mg/ml) and incubated for 5 minutes at room temperature to facilitate the removal of the ectodermal epithelia. After washing in medium, the tissues were cultured on gelatin-coated 24-well dishes. Tissues that had been separated during the dissection were aggregated on the tissue culture dish. The cultures were maintained in α-MEM (Gibco) supplemented with 15% heatinactivated horse serum (Gibco), 2.5% chick embryo extract and 1% penicillin/Streptomycin solution, in vitro for 5 days in 5% CO2 at 37°C. Chick embryo extract was prepared as follows: 9- to 11-day chick embryos were rinsed in PBS and then forced through a 60 ml syringe. An equal volume of α-MEM was added and incubated for 1 hour on ice. After removing the debris by centrifugation at 25,000 revs/minute, the supernatant was sterile filtered and kept frozen at −20°C until used. RNA isolation and reverse transcription At the end of the culture period, medium was aspirated and RNA was prepared from the explants using the method described by Chomczynski and Sacchi (1987). Tissues were lysed in 100 µl lysis buffer (25 g guanidinium thiocyanate, 1.76 ml 0.75 M NaCitrate, pH 7, 2.64 ml 10% Sarkosyl, 38 µl β-mercaptoethanol, 29.3 ml H2O) and transferred to a 1.5 ml centrifuge tube, 10 µl 2 M sodium acetate, pH 4 and 100 µl water saturated phenol were added and mixed, then 20 µl Nt Nc Dermatome Myotome Sclerotome Dermomyotome Sclerotome Epithelial Somite 5 day culture; RT-PCR analysis Fig. 1. Somite maturation and experimental scheme. The diagram on the left depicts the axis of a stage 11+ chick embryo with rostral at the top and caudal at the bottom. The roman numerals indicate the somite stage according to Ordahl, 1993; the last somite formed from the presegmented mesoderm (=psm) being somite I. The diagrams in the middle of the figure depict transverse sections through different axial levels of the embryo. Somites are immature in caudal regions of the embryo, and are progressively more mature in rostral regions. Somites I-III consist of a columnar epithelia and are referred to as epithelial or immature somites within this text. Somite V contains two morphologically distinct groups of cells; dermomyotome and sclerotome. Somite XI contains three morphologically distinct cell types; dermatome, myotome and sclerotome. The experimental design is outlined at the bottom right; unless otherwise stated somites I-III were dissected and cultured for 5 days either alone or in the presence of axial tissues. The induction of skeletal muscle-specific genes was examined by RT-PCR. Axial tissues induce myogenic bHLH genes chloroform/iso-amylalcohol (49:1) was added, mixed and incubated on ice for 15 minutes. Following centrifugation at 4°C, the supernatant was precipitated with 240 µl ethanol in a new tube. Glycogen was included as a carrier (1 µl RNAase-free glycogen, 20 µg/µl, Boehringer Mannheim). After precipitation at −80°C for at least 1 hour, the RNA was pelleted by centrifugation at 4°C for 20 minutes, washed with 80% ethanol, air dried and resuspended in 50 µl buffer (40 mM Tris, ph 8.0; 10 mM NaCl; 6 mM MgCl2) containing 0.5 µl DNAase (RNAase-free DNAase, Boehringer Mannheim, 40 U/ml) and 0.1 µl RNAase inhibitor (USB, 120 U/µl). This was incubated for 1 hour at 37°C, then extracted with Tris-buffered phenol/chloroform and precipitated by adding 1/10 volumes of 3 M sodium acetate, pH 4, and 2.5 volumes ethanol at −80°C. After centrifugation, the RNA pellet was washed twice with 80% ethanol, air dried, dissolved in 10 µl DEPC-treated H2O and kept at −80°C. Before cDNA synthesis, the RNA was incubated at 65°C for 3 minutes then immediately cooled on ice, 5 µl were added to 25 µl Reverse Transcription cocktail, containing 1× transcription buffer (as provided by BRL), 0.5 mM of each dNTP, 200 ng of a random hexamer primer, 0.1 µl RNAase inhibitor, 3.3 mM DTT and 200 U MoMuLV-Reverse Transcriptase (BRL) and incubated at 42°C for 1 hour. The cDNA was kept at −20°C until used for PCR analysis. PCR amplification 1 µl of cDNA was used as template for PCR amplification. Each 50 µl reaction contained 50 mM KCl; 10 mM Tris/HCl, pH 7; 1.5 mM MgCl2, 0.01% gelatin; 200 mM of each dNTP; 0.1 µCi α32P-dCTP (3000 Ci/mMole); 500 ng of the appropriate primers and 1 U Taq polymerase (Boehringer Mannheim). Myf-5 and SHH cDNAs were amplified in the presence of 5% formamide. After an initial denaturation step of 93°C for 3 minutes, the reactions were cycled between 93°C (30 seconds), 60°C (30 sec) and 72°C (1 minute) in a MJ Research thermocycler. The reaction products for MyoD, Myf-5, Myogenin, Myosin Heavy Chain, Twist, choline acetyl-transferase and Sonic hedgehog were amplified in 30 cycles while the product of GAPDH was amplified in 23 cycles. It was determined that at 23 cycles the PCR for GAPDH was in the linear range of amplification. We chose 30 cycles of amplification for the other cDNAs to detect trace amounts of gene products which were induced during in vitro culture. Depending on the amount of template in the culture, 30 cycles amplification of the myogenic bHLH gene products were usually just within the linear range. 5 µl of each PCR reaction was analyzed on a 6% polyacrylamide gel, which was dried and exposed to X-ray film at −20°C for 16 hours. The following primers were used: MyoD (Lin et al., 1989, nt 620-639) 5′-CGTGAGCAGGAGGATGCATA-3′, (nt 864-883) 5′-GGGACATGTGGAGTTGTCTG-3′; Myogenin (Fujisawa-Sehara et al., 1990, nt 435-453) 5′ AGCCTCAACCAGCAGGAG-3′, (nt 694-713) 5′ TGCGCCAGCTCAGTTTTGGA-3′; Myf-5 (Saitoh et al., 1993, nt 283-302) 5′-CTGAGGAAGAGGAACACGTC-3′, (nt 453-434) 5′AGGTCTCGAATGCTTGGTTC-3′; Myosin Heavy Chain (Kavinsky et al., 1983, nt 392-411) 5′-GATCCAGCTGAGCCATGCCA-3′, (nt 1008-989) 5′-GCTTCTGCTCAGCATCAACC-3′; Twist (Doug Spicer, unpublished) 5′-TGTTATCTAGGGCTCTTGCCGG-3′, 5′GCAGAGCGACGAGCTGGACTC-3′; Sonic hedgehog (Riddle et al., 1993) 5′-TGGAAGATATGAAGGGAAGA-3′, 5′CTGAGTTTTCTGCTTTGACG-3′; GAPDH (Dugaiczyk et al., 1983, nt 680-699) 5′-AGTCATCCCTGAGCTGAATG-3′, (nt 9901009) 5′-AGGATCAAGTCCACAACACG-3′. The expected product sizes are as follows: MyoD, 280 nt; Myf-5, 174 nt; Myogenin, 284 nt; MHC, 616 nt; Twist, 150 nt; Sonic hedgehog, 395 nt; GAPDH, 330 nt. In all cases, PCR amplification was shown to be dependent on reverse transcription of RNA templates and on the presence of both forward and reverse primers in the PCR reaction. The identity of each of the PCR products was confirmed by restriction enzyme digestion and, in each case, the expected size fragments were obtained. 653 RESULTS We have employed an in vitro assay to study cell specification in the developing somite with the aim of characterizing inducers of skeletal muscle differentiation. Several studies have demonstrated that somitic cells express immunohistochemically detectable muscle actin and myosin in vitro if cocultured with neural tube (Kenny-Mobbs and Thorogood, 1987; Vivarelli and Cossu, 1986; Buffinger and Stockdale, 1994; Stern and Hauschka, 1994). However, it is not clear from these studies whether signals from the neural tube are necessary for the initial specification of myogenic cells or rather for the differentiation of already determined muscle progenitor cells. Because MyoD and Myf-5 are known to be expressed in proliferating myoblasts prior to their differentiation into skeletal muscle (Tapscott et al., 1988; Braun et al. 1989), these myogenic transcription factors are the earliest available markers of myogenic precursor cells. Therefore, we monitored the expression of MyoD and Myf-5 to assay for the presence of myogenic cells, and myogenin and myosin heavy chain (MHC, embryonic fast form) to assay for muscle differentiation in chick somites co-cultivated with axial tissues (neural tube, floor plate and notochord). If signals from axial tissues were necessary to either induce myogenic precursor cells or maintain their viability, then we expected that no myogenic markers would be expressed in somites cultured in the absence of axial tissues. If, however, axial tissues were necessary solely for differentiation of myogenic precursors, then somites cultured in the absence of axial tissues would express MyoD and/or Myf-5, but fail to express muscle differentiation genes. The neural tube/floor plate/notochord complex promotes myogenic bHLH and myosin heavy chain gene expression The three most caudal somites (somites I-III) of a HamburgerHamilton (HH) stage 12+ chick embryo (containing 17 somites, Hamburger and Hamilton, 1951) were isolated and cultured in the absence or presence of the adjacent neural tube/floor plate/notochord complex. When these somites were isolated and cultured for 5 days in the absence of axial tissues, MyoD, Myf-5, myogenin, and MHC transcripts were not detectable (Fig. 2, lane 1). In contrast, if somites I-III from the contralateral side of the same embryo were cultured in the presence of the adjacent neural tube/floor plate/notochord complex, expression of both myogenic lineage markers (MyoD and Myf-5) and differentiation markers (myogenin and MHC) was observed (Fig. 2, lane 2). No skeletal muscle gene expression was detected in the neural tube or the notochord (data not shown), indicating that skeletal muscle formation in these cultures required the presence of somitic tissue. Furthermore, co-culture of quail somites (I-III) with chick axial tissues resulted in the induction of quail but not chick skeletal muscle (data not shown), indicating that all skeletal muscle in these cultures was somite-derived. These findings indicate that signals from axial tissues are necessary to promote the expression of both myogenic lineage markers (MyoD and Myf-5) and differentiation markers (myogenin and myosin heavy chain). Interestingly, expression of MyoD can first be detected in somite II of stage 11-12 quail embryos by in situ hybridization (Pownall and Emerson, 1992; 654 A. E. Münsterberg and A. B. Lassar cultures, the gene Twist is expressed mainly in somitic mesoderm and very weakly in notochord (data not shown). Readily detectable levels of both Twist and GAPDH in somites cultured alone (Fig. 2, lane 1) attest to the viability of somitic cells cultured in the absence of axial tissues. However, this data does not rule out the possibility that some somitic lineages may selectively die in the absence of axial tissues. The increased amount of Twist expression in somites cultivated with axial tissues (Fig. 2, lane 2) could be due to either proliferation of somitic cells or up-regulation of Twist expression in sclerotomal cells (Hopwood et at., 1989b; Wolf et al., 1991). Signals from both the neural tube and the ventral midline tissues (floor plate and notochord) are necessary for somite myogenesis in vitro To investigate which axial tissues are required for somitic muscle differentiation, we dissected the neural tube away from the floor plate/notochord complex. We subsequently co-cultivated immature somites of stage 10+/11 embryos with either neural tube lacking floor plate, neural tube plus floor Fig. 2. The neural tube/ floor plate/ notochord complex is necessary for myogenic bHLH gene expression. Somites I-III of a stage 12+ (17 somite) chick embryo were dissected either alone or together with the neighboring neural tube/floor plate/notochord complex as diagrammed above each lane. The endodermal and ectodermal epithelia were removed, and the tissues were cultured for 5 days in the absence (lane 1) or the presence (lane 2) of the neural tube/floor plate/notochord complex. At the end of the culture period, the tissues were harvested and assayed for the expression levels of MyoD, Myf5, Myogenin, Myosin Heavy Chain (MHC), the bHLH gene Twist, and GAPDH by Reverse Transcriptase-PCR analysis (RT-PCR). The lower amounts of GAPDH in the culture containing somites only (lane 1) reflect a lower amount of tissue in this culture, where neural tube and notochord are absent. Neural tube and notochord do not express detectable levels of MyoD, Myf-5, Myogenin or Myosin Heavy Chain (data not shown). Similar results were obtained with 34 embryos ranging from stage 10 to stage 13. Barth and Ivarie, 1994), and is detectable by RT-PCR at extremely low levels in chick somites I-III which have not been cultured in vitro (data not shown). The absence of detectable MyoD in chick somites I-III cultured alone indicates that the initial expression of MyoD in these somites is not maintained in the absence of axial tissues (see also Bober et al. 1994). Taken together, these results suggest that, in the presence of axial tissues, the expression of MyoD is both maintained and significantly up-regulated. Previous studies have suggested that either the stability of the somite, or the viability of somitic cells may require the presence of adjacent axial tissues (Lipton and Jacobson, 1974; Teillet and Le Douarin, 1983; Rong et al. 1992). To monitor the viability of somites cultured in vitro, we assayed the expression of GAPDH and Twist in somites cultured either alone or in the presence of axial tissues. In these explant Fig. 3. Signals from both dorsal neural tube and floor plate/ notochord are necessary for myogenesis of somites I-III in stage 11 embryos. Somites I-III, dissected from a stage 10+ embryo were cultured in the presence of neural tube alone (lane 1), or in the presence of neural tube, floor plate and notochord (lane 2). Somites IV-VI, dissected from a stage 10+ embryo were cultured with floor plate and notochord (lane 3). Somites I-III, dissected from a stage 11 embryo were cultured either in the presence of neural tube alone (lane 4), or in the presence of neural tube and floor plate (lane 5). The tissues present in each culture are diagrammed above each lane. Note that in these and in subsequent dissections the neural tube was transected along its dorsal-ventral axis such that ipsilateral and contralateral somites were cultured with comparable neural tissue. Expression levels of MyoD, Myf-5, Myosin Heavy Chain (MHC), Sonic hedgehog (SHH) and GAPDH were assayed by RT-PCR analysis. In these explant cultures, SHH is a specific marker for floor plate and notochord. Similar results were obtained with eight (lanes 1 and 2), three (lane 3), or four independent embryos (lanes 4 and 5). Axial tissues induce myogenic bHLH genes plate/notochord, or floor plate/notochord in the absence of neural tube (Fig. 3). As shown in the previous experiment, robust activation of myogenic bHLH genes and skeletal muscle differentiation was observed in somites that had been cultured with neural tube plus floor plate and notochord (Fig. 3, lane 2). If, however, the somites were cultured either with neural tube alone (Fig. 3, lane 1), or with the floor plate/notochord complex, alone (Fig. 3, lane 3), only trace levels of skeletal muscle gene expression were observed. In these tissue explants, Sonic hedgehog (SHH) is a specific marker for both floor plate and notochord (Echelard et al., 1993; Krauss et al., 1993; Riddle et al., 1993; Roelink et al., 1994). Thus SHH expression was monitored to assay for the presence of these ventral midline tissues in the various cultures. This experiment indicates that, under the culture conditions employed, neither the neural tube nor the ventral midline tissues (floor plate and notochord) were sufficient to support myogenesis of immature somites from stage 10+/11 embryos. However, the presence of both neural tube and ventral midline tissues was able to promote skeletal muscle differentiation in vitro of similar stage somites. The notochord has been demonstrated to induce the floor plate in the ventral neural tube (van Straaten et al. 1988; Smith and Schoenwolf, 1989; Placzek et al., 1990, 1993; Yamada et al., 1991). Furthermore, the notochord shares several inducing capacities with the floor plate (Yamada et al., 1991, 1993; Pourquié et al., 1993; Placzek et al. 1993). Thus, we reasoned that signals from the floor plate/notochord complex could be emitted by one or both of these tissues. To clarify if the floor 655 plate itself was able to promote somitic myogenesis, somites I-III from stage 11 embryos were cultured with neural tube, after removal of the underlying notochord. After 5 days in culture, this neural tube, which contained floor plate as shown by high level expression of SHH, was capable of inducing skeletal muscle in somitic tissue (Fig. 3, lane 5). Thus somites I-III from a stage 11 embryo can be induced to form skeletal muscle by a combination of signals derived from the neural tube and floor plate. The requirements for somite myogenesis in vitro differ along the anteroposterior axis of the embryo In vertebrate embryos, a developmental gradient exists, with rostral regions of the axis being developmentally more advanced than caudal regions. Skeletal muscle differentiation of somites, independent of axial tissues, is observed first in rostral regions of the embryo and later in caudal regions (Rong et al., 1992; Borman et al., 1994; Buffinger and Stockdale, 1994; Stern and Hauschka, 1994). We therefore analyzed whether the tissue requirements for somite myogenesis in vitro differ in somites taken from different axial levels. Paraxial mesoderm was explanted in blocks of three somites from a stage 11− embryo (containing 12 somites), starting caudally with the presegmented mesoderm and going successively more rostral. The somites were cultured either alone (Fig. 4A, lanes 1-4), or in the presence of neural tube excluding the floor plate (Fig. 4A, lanes 5-8). In the absence of neural tube, the somites at all axial levels examined did not differentiate into skeletal muscle; MyoD was either undetectable or Fig. 4. (A) Signals from neural tube alone are sufficient to induce myogenesis in rostral but not caudal regions of a stage 11− embryo. Somites were dissected along the rostrocaudal axis of a stage 11− embryo (12 somite) in blocks of 3 (presegmented mesoderm, lanes 1 and 5; somites I-III, lanes 2 and 6; somites IV-VI, lanes 3 and 7; somites VII-IX, lanes 4 and 8) and somites were either cultured alone (lanes 1-4) or in the presence of adjacent neural tube lacking floor plate (lanes 5-8). The tissues present in each culture are diagrammed above each lane. Expression levels of MyoD, Myf5, Myosin heavy chain (MHC), the bHLH gene Twist, Sonic Hedgehog (SHH) and GAPDH were assayed by RT-PCR analysis. In these explant cultures SHH is a specific marker for floor plate and notochord. Similar results were obtained in four independent experiments. (B) Axial tissues are necessary to initiate but not to maintain the myogenic program during somite maturation. Somites XIIXIV were dissected from a stage 16 (28 somite) embryo and either harvested for RNA analysis without culture (lane 1), or cultured in the absence (lane 2) or presence (lane 3) of the neural tube/floor plate/notochord complex. The tissues present in each culture are diagrammed above each lane. Expression levels of MyoD, Myogenin, Myosin Heavy Chain (MHC) and GAPDH were assayed by RT-PCR analysis. 656 A. E. Münsterberg and A. B. Lassar expressed at very low levels, and Myf-5 and MHC transcripts were not detected (Fig. 4A, lanes 1-4). In caudal regions of the embryo, the presence of the neural tube alone was not sufficient for somitic myogenesis (Fig. 4A, lanes 5 and 6). In contrast, in more rostral regions of the embryo the presence of neural tube resulted in robust induction of skeletal muscle (Fig. 4A, lanes 7 and 8). The extremely low levels of Sonic hedgehog expression in all the above explants suggest that myogenesis in cultures containing neural tube from rostral regions of the embryo was not due to the presence of floor plate in these samples. These findings suggest that, as somites mature, myogenesis of the somite (in vitro) becomes independent of the ventral midline tissues, but still requires interaction with the neural tube. As somites mature further, myogenesis in vitro becomes independent of axial tissues (Kenny-Mobbs and Thorogood, 1987; Vivarelli and Cossu, 1986; Buffinger and Stockdale, 1994; Stern and Hauschka, 1994). We observed equivalent levels of myogenesis in somites XII-XIV isolated from a stage 16 embryo cultured in the absence or presence of axial tissues (Fig. 4B, lanes 2 and 3, respectively). Therefore, these somites can operationally be defined as specified for myogenesis. Interestingly, the myogenic regulators MyoD and myogenin were expressed in these somites at low levels at the time of dissection. Transcripts for MHC were below levels of detection (Fig. 4B, lane 1). Thus, somites that are specified for myogenesis have received sufficient signals to allow up-regulation of myogenic bHLH genes and the expression of muscle differentiation markers in the absence of further induction. Taken together, these observations indicate that immature paraxial mesoderm (i.e., presegmented plate and somites I-III in stage 11− embryos) requires at least two signals for myogenesis. In vitro these signals can be provided by the floor plate and more dorsal regions of the neural tube, respectively (and perhaps by other tissues in the embryo). Slightly more mature somites (somite IV-IX in stage 11− embryos) only require signals from the neural tube for myogenesis in vitro. In still more mature somites, myogenesis becomes independent of the adjacent axial tissues (somites XII-XIV in stage 16 embryos). The competence of somites to initiate myogenesis differs along the axis of the embryo The ability of neural tube lacking floor plate to induce myogenesis in rostral (somites IV-IX) but not caudal (presegmented mesoderm - somite III) paraxial mesoderm is consistent with a number of possibilities. Either somites IV-IX (from a stage 11− embryo) have different intrinsic signaling requirements than more caudal paraxial mesoderm or they have progressed in their maturation such that they can respond to a signal(s) from neural tube alone. In addition, it is conceivable that neural tube adjacent to somites IV-IX has different signaling properties than neural tube adjacent to more caudal paraxial mesoderm, allowing the former to induce skeletal muscle in the absence of floor plate and notochord. To address whether it is the mesoderm or the neural tube that differs in its capacity to support myogenesis in caudal versus rostral paraxial mesoderm, we recombined paraxial mesoderm and neural tube from these different axial levels. Robust myogenesis was observed in somites VII-IX (taken from a stage 10+ embryo) that had been cultured with either adjacent neural tube or neural tube that had been adjacent to presegmented mesoderm – somite I (Fig. 5, lanes 3 and 4). In contrast, extremely low levels of myogenesis were observed in caudal paraxial mesoderm (presegmented mesoderm – somite I from the same embryo) that had been cultured with either adjacent neural tube or neural tube from axial levels adjacent to somites VII-IX (Fig. 5, lanes 1 and 2). The trace levels of MyoD expression observed in these latter cultures can probably be attributed to the presence of a few floor plate cells in these cultures, as evidenced by the low level of Sonic hedgehog expression. Importantly, paraxial mesoderm from either of these axial levels (from this stage embryo) did not differentiate into skeletal muscle in the absence of axial tissues (data not shown; Rong et al., 1992; Borman et. al., 1994; Buffinger and Stockdale, 1994; Stern and Hauschka, 1994). Thus somites VII-IX taken from a stage 10+ embryo were competent to differentiate into skeletal muscle in vitro in response to a signal from the neural tube, whereas more caudal Fig. 5. The competence of somites to initiate myogenesis in response to a neural tube signal changes along the axis of a stage 10+ embryo. Presegmented mesoderm and somites I were dissected from a stage 10+ embryo and either cultured with the adjacent neural tube (lane 1) or with more rostral neural tube (adjacent to somites VII-IX) from the same embryo (lane 2). Somites VII-IX were dissected from the same embryo and either cultured with the adjacent neural tube (lane 4) or with neural tube from more caudal regions (adjacent to presegmented mesoderm-somite I, lane 3). The origin of somitic mesoderm and neural tubes in each co-culture is diagrammed above each lane (tissues from caudal axial levels are depicted as white, tissues from rostral axial levels are depicted as stippled). Expression levels of MyoD, Myosin Heavy Chain (MHC), Sonic hedgehog (SHH), and GAPDH were assayed by RT-PCR analysis. In these explant cultures, Sonic hedgehog (SHH) is a marker for both notochord and floor plate. The absence of SHH expression in lanes 3 and 4 indicates that these explants contained no floor plate cells. The low amount of SHH PCR product in lanes 1 and 2 indicates the presence of a few floor plate cells in these explants. Identical results were obtained in four independent experiments (lanes 1 and 4) or in duplicate (lanes 2 and 3). Axial tissues induce myogenic bHLH genes paraxial mesoderm lacked this ability; neural tube from both axial levels could provide this signal. A Series Signaling NT 3 657 M DISCUSSION In this study, we demonstrate that signals from both the neural tube and floor plate together support the expression of the myogenic bHLH regulators in the somite in vitro. This finding is consistent with a requirement for signals from axial tissues in either mediating the initial induction of somitic cells to become myoblasts or in supporting the proliferation and survival of such determined cells. Prior embryological manipulations have shown that differentiation of the axial musculature in the chicken embryo requires proximity between the somite and the neural tube (Christ et al., 1992; Rong et al., 1992). Our work has confirmed and extended this analysis to demonstrate that stable expression of the myogenic bHLH regulatory family of transcription factors requires at least two signals. In our in vitro co-culture system, these signals can be provided by the floor plate and regions of the neural tube dorsal to the floor plate. We provide evidence to suggest that the competence of somites to respond to the signal from the neural tube (in the absence of floor plate) changes as somites mature. Therefore, we propose that signals from neural tube and floor plate act directly upon the somite and together induce skeletal muscle formation. Previous studies have documented the importance of notochord and floor plate in the induction of sclerotome (Grobstein and Holtzer 1955; Lash et al. 1957; Hall 1977, Brand-Saberi et al. 1993; Dietrich et al., 1993; Koseki et al. 1993; Pourquié et al., 1993). This study, together with other recent reports (Rong et al. 1993; Buffinger and Stockdale 1994; Stern and Hauschka 1994), suggests that ventral midline tissues (floor plate and notochord) may also play a role in the formation of somitic skeletal muscle. Parallel signals from the neural tube and floor plate/notochord synergistically activate the myogenic program Skeletal myogenesis in either presegmented plate or somites IIII (isolated from a stage 11− or younger chick embryo) required co-cultivation of the paraxial mesoderm with both neural tube dorsal to the floor plate and the floor plate itself. The requirement for two different regions of the neural tube for somitic myogenesis in vitro is consistent with three possible signaling schemes. Either signals from the floor plate induce a secondary signal in more dorsal regions of the neural tube, which in turn activates somite myogenesis (Series Signaling; see Fig. 6A) or signals from the floor plate act together with signals from the neural tube to synergistically activate somite myogenesis (Parallel Signaling; see Fig. 6B). In the former, the signals act in a dependent sequential cascade; in the latter the signals are unlinked and work independently. Lastly, a combination of the above two models is possible; in this case, signals from the floor plate induce a secondary signal in the neural tube, which then acts in parallel with other signals from the floor plate to induce somite myogenesis (Series + Parallel Signaling; see Fig. 6C). In contrast to presegmented mesoderm and somites I-III from stage 11− embryos, which require neural tube plus ventral midline tissues to form skeletal muscle, somites IV-IX from the 2 1 B Parallel Signaling NT 1 C Series + Parallel Signaling NT Fp Nc 3 Fp 2 Nc 3 2 1 M Fp M 4 Nc Fig. 6. Multiple signals are involved in somitic myogenesis. (A) Series signaling. Signal 1 from the notochord induces floor plate in the ventral neural tube. Signal 2 from the floor plate travels within the neural tube to affect the production of Signal 3. Signal 3 from the neural tube induces skeletal muscle formation in the adjacent somite. (B) Parallel signaling. Signal 1 from the notochord induces floor plate in the ventral neural tube. Signal 2 from the floor plate travels directly to the somite. Signal 3 from the neural tube travels directly to the somite. The convergence of signals 2 and 3 within the somite induces skeletal muscle formation. (C) Series + parallel signaling. Signal 1 from the notochord induces floor plate in the ventral neural tube. Signal 2 from the floor plate travels within the neural tube to affect the production of signal 3. Signal 4 from the floor plate travels directly to the somite. The convergence of signals 3 and 4 within the somite induces skeletal muscle formation. Our experiments do not rule out the possibility that signals 2 and 4 could also be emitted directly by the notochord. same stage embryos form skeletal muscle if co-cultured solely with neural tube lacking floor plate. We analyzed whether the paraxial mesoderm or the neural tube differs in its capacity to support myogenesis at different axial levels (Fig. 5). Our data suggest that the competence of paraxial mesoderm to respond to a signal from the neural tube is different at varying axial levels. It is unclear what signals in the embryo have promoted the com- 658 A. E. Münsterberg and A. B. Lassar petence of somites IV-IX to respond to a signal from the neural tube. However, we have found that the floor plate/notochord can provide this signal to somites I-III from a similar stage embryo (Fig. 3). Taken together, these findings imply that signals from both the neural tube and ventral midline tissues are required for somitic myogenesis. In addition, the data are most consistent with a parallel signaling scenario (see Fig. 6B) in which a signal from the floor plate and notochord (see below) is transmitted directly to the somite; this signal renders these cells responsive to muscle-inducing signals from the neural tube. Therefore, we propose that a combination of independent signals from the neural tube and the floor plate/notochord act directly on the paraxial mesoderm to induce myogenesis. As a result of these interactions, the myogenic bHLH genes are stably activated. Stable activation of myogenic bHLH regulators correlates with somitic muscle differentiation that is independent of the axial tissues, suggesting that inductive signals from axial tissues are necessary for the initiation but not the maintenance of somite myogenesis. Is the notochord involved in skeletal myogenesis? Two groups have recently shown that notochord can induce a small but detectable level of myogenesis in somites II-III, but not in presegmented mesoderm or somite I from stage 11-13 chick embryos (Buffinger and Stockdale, 1994; Stern and Hauschka, 1994). These observations, together with previous observations (Rong et al. 1992), indicate that notochord can induce skeletal muscle in some paraxial mesoderm (somite II or older). This is in contrast to our own finding where somites I-III of stage 11 or younger embryos required two signals (from the neural tube and the ventral midline tissues) for robust myogenesis (Fig. 3). It is not clear whether this difference is due to different culture conditions, or whether a small amount of notochord-dependent myogenesis was below levels of detection by RT-PCR. However, the inability of notochord to induce myogenesis in presegmented plate (Buffinger and Stockdale, 1994; Stern and Hauschka, 1994) is consistent with our findings that two signals are required for somite myogenesis, and suggests that induction of muscle by notochord may only occur in somites that have received another signal. In our in vitro culture system, somitic skeletal muscle is induced by factors present in the neural tube and the floor plate. It seems that notochord is not required (Fig. 3). However, numerous studies have documented that the differentiation of floor plate depends on signals from the notochord (van Straaten et al., 1989; Placzek et al., 1990, 1993; Yamada et al., 1991, 1993). Therefore, the apparent requirement of floor plate signals for somitic muscle formation in vitro implies that notochord may also be involved in myotome formation in vivo. Furthermore, the documented muscle-inducing capacity of either floor plate (this study) or notochord (Rong et al., 1992: Buffinger and Stockdale, 1994; Stern and Hauschka, 1994) suggest that both these tissues may play a direct role in promoting somite myogenesis in the embryo. However, it is not clear whether muscleinducing signals from either one or both of these ventral midline tissues are necessary for somite myogenesis. In light of the many signaling properties common to both notochord and floor plate (Yamada et al., 1991, 1993; Echelard et al., 1993; Pourquié et al., 1993; Placzek et al. 1993; Riddle et al., 1993; Roelink et al., 1994), it seems plausible that the muscle-inducing activities of these tissues may be similar, if not identical. A prediction of all the models schematized in Fig. 6 is that removal of notochord, prior to induction of floor plate, should inhibit the formation of myotome in vivo. Whereas it was demonstrated that sclerotome formation in the somite is disrupted following notochord removal (Dietrich et al., 1993; Koseki et al., 1993; Goulding et al., 1994), myotome formation was reported to occur in somites that had fused beneath the remaining neural tube (van Straaten and Hekking 1991; Goulding et al., 1994). Interestingly, Goulding and colleagues noted that MyoD expression did not occur in somites that were positioned at a distance from the residual notochord, suggesting that signals from the notochord may be necessary for MyoD induction in vivo (Goulding et al., 1994). Myotome formation in vivo, in the absence of notochord, is consistent with a number of explanations: (1) possible signaling by the notochord to paraxial mesoderm prior to removal of the notochord, (2) differentiation of floor plate in the absence of underlying notochord (described in Artinger and BronnerFraser, 1993) possibly mediated by homeogenetic induction of floor plate within the plane of the neural tube (Placzek et al., 1993), (3) signaling of floor plate and notochord to axial levels that are anterior and posterior to the transected ends of the notochord, and (4) another signaling source in the embryo that shares the muscle promoting properties of the floor plate/notochord complex such that these signals would be redundant in the embryo but unique in explant cultures containing only somite and axial tissues. The relative position of somite cells with respect to neural tube, floor plate and notochord may determine cell fate There is a wealth of experimental evidence indicating that sclerotome formation in the somite is induced by signals from the axial tissues (Grobstein and Holtzer, 1955; Lash et al., 1957; Hall, 1977, Brand-Saberi et al., 1993). These classical studies, together with more recent grafting experiments in vivo (Pourquié et al., 1993), and the analysis of gene expression patterns in mutant mouse embryos (Dietrich et al., 1993) surgically manipulated chick embryos (Brand-Saberi et al., 1993; Koseki et al., 1993) and explant culture (Fan and Tessier-Lavigne, 1994), have implied a role for the floor plate and notochord in sclerotome formation. Our own experiments indicate that specification of somitic cells into myotome results from the combined action of at least two signals; from the floor plate/notochord and regions dorsal to the floor plate in the neural tube. Dermatome formation may either result as a default pathway for somitic cells in the absence of signals from axial tissues, or may be actively induced by interaction with the overlying epidermis. Thus specification of somitic cell fate into either dermatome, myotome or sclerotome may in part be dictated by the position of somitic cells relative to the neural tube, floor plate and notochord (see also Pourquié et al., 1993; Fan and Tessier-Lavigne, 1994). Implantation of either an ectopic floor plate or notochord between the neural tube and the paraxial mesoderm induces ectopic sclerotome while inhibiting the formation of myotome (Pourquié et al. 1993). These authors suggested that signals from the floor plate and notochord prevented myotome formation by inducing ventral cell types (i.e. sclerotome), and therefore it seems paradoxical that the ventral midline tissues would play a positive role in myotome differentiation (this Axial tissues induce myogenic bHLH genes report, Buffinger and Stockdale, 1994; Stern and Hauschka, 1994). A possible resolution to this paradox lies in the fact that the ectopic notochord/floor plate in these experiments was implanted between the neural tube and the paraxial mesoderm and therefore may have interfered with signaling between these two tissues. It is not clear whether different signaling molecules or a gradient of the same single signaling molecule emanating from the floor plate/ notochord complex promotes formation of the myotome versus the sclerotome. Furthermore, it is possible that myotome formation requires cell-cell signaling within the somite, for example from induced sclerotomal cells. In summary, it seems well established that somitic cell fate is dictated by cues from the neural tube, floor plate and notochord. However, clarification of whether these signals are expressed in an interdependent manner in the axial tissues, and/or whether they induce a further cascade of signals in somitic tissue must await characterization of the molecules involved. We thank Hazel Sive, Cliff Tabin, Tom Schultheiss and Mark Mercola for their comments on previous versions of this manuscript; the anonymous reviewers of this manuscript for their insightful suggestions; Tom Jessell, Sam Pfaff, Toshiya Yamada and Doug Spicer for sharing reagents and unpublished sequence information with us, and Nicholas Buffinger, Charlie Emerson, Steve Hauschka, Howard Stern and Frank Stockdale for sharing unpublished information with us. This work was supported by grants to A. B. L. from the National Science Foundation, the Lucille P. Markey Charitable Trust, the Muscular Dystrophy Association, The Council for Tobacco Research USA, and a Basil O’Connor Award no. 1 FY93-0848 from the March of Dimes Birth Defects Foundation. A. B. L. was a Lucille P. Markey Scholar. A. E. M. was supported by fellowships from the Human Frontiers Science Program Organization and the Muscular Dystrophy Association. REFERENCES Aoyama, H. and Asamoto, K. (1988). Determination of somite cells: independence of cell differentiation and morphogenesis. Development 104, 15-28. Artinger, K. B. and Bonner-Fraser, M. (1993). Delayed formation of the floor plate after ablation of the avian notochord. Neuron 11, 1147-1161. Avery, G., Chow, M. and Holtzer, H. (1956). An experimental analysis of the development of the spinal column. J. Exp. Zool. 132, 409-425. Barth, J. and Ivarie, R. (1994). Polyvinyl alcohol enhances detection of low abundance transcripts in early stage quail embryos in a nonradioactive whole mount in situ hybridization technique. Biotechniques 17, 324-327. Bober, E., Lyons, G. E., Braun, T., Cusso, G. and Buckingham, M. (1991). The muscle regulatory gene, Myf-6, has a biphasic pattern of expression during early mouse development. J. Cell Biol. 113, 1255-1265. Bober, E., Brand-Saberi, B., Ebensperger, C., Wilting, J., Balling, R., Paterson, B. M., Arnold, H.-H. and Christ, B. (1994). Initial steps of myogenesis in somites are independent of influence from axial structures. Development 120, 3073-3082. Borman, W. H., Urlakis Jr., K. J. and Yorde, D. E. (1994). Analysis of the in vivo myogenic status of chick somites by desmin expression in vitro. Developmental Dynamics 199, 268-279. Brand-Saberi, B., Ebensperger, C., Wilting, J., Balling, R. and Christ, B. (1993). The ventralizing effect of the notochord on somite differentiation in chick embryos. Anat. Embryol. 188, 239-245. Braun, T., Bober, E., Buschhausen-Denker, G., Kohtz, S., Grzeschik, K-H. and Arnold, H. H. (1989). Differentential expression of myogenic determination genes in muscle cells: possible autoactivation by the Myf gene products. EMBO J. 8, 3617-3625. 659 Buckingham, M. (1992). Making muscle in mammals. Trends in Genetics 8, 144-149. Buffinger, N. and Stockdale, F. (1994). Myogenic specification in somites: induction by axial structures. Development 120, 1443-1452. Charles de la Brousse, F. and Emerson Jr., C. P. (1990). Localized expression of a myogenic regulatory gene, qmf1, in the somite dermatome of avian embryos. Genes and Develop. 4, 567-581. Christ, B., Brand-Saberi, B., Grim M. and Wilting, J. (1992). Local signalling in dermomyotomal cell type specification. Anat. Embryol. 186, 505-510. Christ, B. and Wilting, J. (1992). From somites to vertebral column. Annals of Anatomy 174, 23-32. Chomczynski, P. and Sacchi, N. (1987). Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal. Biochem. 162, 156-159. Dietrich, S., Schubert, F. R. and Gruss, P. (1993). Altered Pax gene expression in murine notochord mutants: the notochord is required to initiate and maintain ventral identity in the somite. Mech. o. Dev. 44, 189-207. Dugaiczyk, A., Haron, J. A., Stone, E. M., Dennison, O. E. Rothblum, K. N. and Schwartz, R. J. (1983). Cloning and sequencing of a deoxyribonucleic acid copy of glyceraldehyde-3-phosphate dehydrogenase messenger ribonucleic acid isolated from chicken muscle. Biochemistry 22, 1605-1613. Echelard, Y., Epstein, D. J., St-Jacues, B., Shen, L., Mohler, J., McMahon, J. A. and McMahon, A. P. (1993). Sonic Hedgehog, a member of a family of putative signaling molecules, is implicated in the regulation of CNS polarity. Cell 75, 1417-1430. Emerson Jr., C. P. (1993). Embryonic signals for skeletal myogenesis: arriving at the beginning. Current Opinion in Cell Biology 5, 1057-1064. Fan, C.-M. and Tessier-Lavigne, M. (1994). Patterning of mammalian somites by surface ectoderm and notochord: evidence for sclerotome induction by a hedgehog homolog. Cell 79, 1-20. Fujisawa-Sehara, A., Nabeshima, Y., Hosoda, Y., Obinata, T. and Nabeshima, Y. I. (1990). Myogenin contains two domains conserved among myogenic factors. J. o. Biological Chemistry 265, 15219-15223. Goulding, M., Lumsden, A. and Paquette, A. J. (1994). Regulation of Pax-3 expression in the dermomyotome and its role in muscle development. Development 120, 957-971. Grobstein, C. and Holtzer, H. (1955). In vitro studies of cartilage induction in mouse somite mesoderm. J.exp.Zoology 128, 333-356. Hall, B. K. (1977). Chondrogenesis of the somitic mesoderm. Springer-Verlag Berlin Heidelberg New York, eds. Hamburger, V. and Hamilton, H. L. (1951). A series of normal stages in the development of the chick embryo. J.Morph. 88, 49-92. Hasty, P., Bradley A., Morris, J. H., Edmondson, D. G., Venuti, J. M., Olson, E. and Klein, W. H. (1993). Muscle deficiency and neonatal death in mice with a targeted mutation in the myogenin gene. Nature 364, 501-506. Hinterberger, T. J., Sassoon, D. A., Rhodes, S. J. and Konienczny, S. F. (1991). Expression of the muscle regulatory factor MRF4 during somite and skeletal myofiber development. Dev. Biol. 147, 144-156. Hopwood, N. D. Pluck, A. and Gurdon, J. B. (1989a). MyoD expression in the forming somites is an early response to mesoderm induction in Xenopus embryos EMBO J. 8, 3409-3417. Hopwood, N. D. Pluck, A. and Gurdon, J. B. (1989b). A Xenopus mRNA related to Drosophila twist is expressed in response to induction in the mesoderm and the neural crest. Cell 59, 893-903. Kavinsky, C. J., Umeda, P. K., Sinha, A. M., Wlzinga, M. W., Tong, S. W., Zak, R., Jakovicic, S. and Rabinowitz, M. (1983). Cloned mRNA sequences for two types of embryonic myosing heavy chains from chick skeletal muscle. J. Biological Chemistry 258, 5196-5205. Kenny-Mobbs, T. and Thorogood, P. (1987). Autonomy of differentiation in avian brachial somites and the influence of adjacent tissues. Development 100, 449-462. Koseki, H., Wallin, J., Wilting, J., Mizutani, Y., Kispert, A., Ebensperger, C., Herrmann, B., Christ, B. and Balling, R. (1993). A role for Pax-1 as a mediator of notochordal signals during the dorsoventral specification of vertebrae. Development 119, 649-660. Krauss, S., Concordet, J. P. and Ingham, P. W. (1993). A functionally conserved homolog of the Drosophila segment polarity gene hh is expressed in tissues with polarizing activity in zebrafish embryos. Cell 75, 1431-1444. Lash, J., Holtzer, S. and Holtzer, H. (1957). An experimental analysis of the development of the spinal column. Exp. Cell Res. 13, 292-303. Lassar, A. B. and Münsterberg, A. E. (1994). Wiring diagrams: regularoty circuits and the control of skeletal myogenesis. Current Opinion in Cell Biology 6, 432-442. 660 A. E. Münsterberg and A. B. Lassar Lin, A.-Y. Dechesne, C. A., Eldridge, J., and Paterson, B. M. (1989). An avian muscle factor related to MyoD1 activates muscle-specific promoters in nonmuscle cells of different germ-layer origin and in BrdU-treated myoblasts. Genes Dev. 3, 986-996. Lipton, B. H. and Jacobson, A. G. (1974). Experimental analysis of the mechanisms of somite morphogenesis. Dev. Biol. 38, 91-103. Nabeshima, Y., Hanaoka, K., Hayasaka, M., Esumi, E., Li, S., Nonaka, I., Nabeshima, Y. (1993). Myogenin gene disruption results in perinatal lethality because of severe muscle defect. Nature 364, 532-535. Olson, E. N. and Klein W. H. (1994). bHLH factors in muscle development: dead lines and commitments, what to leave in and what to leave out. Genes Dev. 8,1-8. Ordahl, C. P. and Le Douarin, N. (1992). Two myogenic lineages within the developing somite. Development 114, 339-353. Ordahl, C. P. (1993). Myogenic lineages within the developing somite. Molecular Basis of Morphogenesis, pp. 165-176. Wiley-Liss, Inc. Ott, M.-O., Bober, E., Lyons, G., Arnold, H., and Buckingham M. (1991). Early expression of the myogenic regulatory gene, myf-5, in precursor cells of skeletal muscle in the mouse embryo. Development 111, 1097-1107. Packard Jr., D. S., and Jacobson, A. G. (1976). The influence of axial structures on chick somite formation. Dev. Biol. 53, 36-48. Placzek, M., Tessier-Lavigne, M., Yamada, T., Jessell,T., and Dodd, J. (1990). Mesodermal control of neural cell identitiy: Floor plate induction by the notochord. Science 250, 985-988. Placzek, M., Jessell, T. M., and Dodd, J. (1993). Induction of floor plate differentiation by contact-dependent, homeogenetic signals. Development 117, 205-218. Pourquié, O., Coltey, M., Teillet, M-A., Ordahl, C., LeDouarin, N. M. (1993). Control of dorsoventral patterning of somitic derivatives by notochord and floor plate. Proc. Natl. Acal. Sci. USA 90, 5242-5246. Pownall, M. E., and Emerson Jr., C. P. (1992). Sequential activation of three myogenic regulatory genes during somite morphogenesis in quail embryos. Dev. Biol. 151, 67-79. Riddle, R. D., Johnson, R. L., Laufer, E., Tabin, C. (1993). Sonic hedgehog mediates the polarizing activity of the ZPA. Cell 75, 1401-1416. Roelink, H., Augsburger, A., Heemskerk, J., Korzh,V., Norlin., s., Ruiz i Altaba, A., Tanabe, Y., Placzek, M., Edlund, T., Jessell, T. M., Dodd, J. (1994). Floor plate and motor neuron induction by vhh-1, a vertebrate homolog of hedgehog expressed by the notochord. Cell 76, 761-775. Rong, P. M., Teillet, M.-A., Ziller, C., and Le Douarin, N. M. (1992). The neural tube/floor plate/notochord complex is necessary for vertebral but not limb and body wall striated muscle differentiation. Development115, 657672. Rudnicki, M. A., Schnegelsberg, P. N. J., Stead, R. H., Braun, T., Arnold, H. H., Jaenisch, R. (1993). MyoD or Myf-5 is required for the formation of skeletal muscle. Cell 75, 1351-1359. Saitoh, O., Fujisawa-Sehara, A., Nabeshima, Y., Periasamy, M. (1993). Expression of myogenic factors in denervated chicken breast muscle: isolation of the chicken Myf-5 gene. Nucleic Acid Res. 21, 2503-2509. Sassoon, D. (1993). Myogenic regulatory factors: dissecting their role and regulation during vertebrate embryogenesis. Dev. Biol. 156, 11-23. Sassoon, D., Lyons, G., Wright, W. E., Lin, V., Lassar, A. B., Weintraub, H. and Buckingham, M. (1989). Expression of two myogenic regulatory factors, myogenin and MyoD1, during mouse embyogenesis. Nature 341, 303-307. Smith, J. L. and Schoenwolf, G. C. (1989). Notochordal induction of cell wedging in the chidk neural plate and its role in neural tube formation. J. Exp. Zoology 250, 49-62. Stern, H. and Hauschka, S. (1994). Neural tube and notochord promote in vitro myogenesis in single somite explant cultures. Dev. Biol. (in press). Tapscott, S. J., Davis, R. L., Thayer, M. J., Cheng, P.-F., Weintraub, H., Lassar, A. B. (1988). MyoD1: a nuclear phosphoprotein requiring a Myc homology region to convert fibroblasts to myoblasts. Science 242, 405-411. Teillet, M.-A. and Le Douarin, N. M. (1983). Consequences of neural tube and notochord excision on the development of the peripheral nervous system in the chick embryos. Dev. Biol. 98, 192-211. van Straaten, H. W. M., Hekking, J. W. M., Wiertz-Hoessels, E. L., Thors, F., Drukker, J. (1988). Effect of the notochord on the differentiation of a floor plate area in the neural tube of the chick embryo. Anat. Embryol. 177, 317-324. van Straaten, H. W. M., Hekking, J. W. M. (1991). Development of floor plate, neurons and axonal autgrowth pattern in the early spinal cord of the notochord-deficient chick embryo. Anat. Embryol. 184, 55-63 Vivarelli, E. and Cossu, G. (1986). Neural control of early myogenic differentiation in cultures of mouse somites. Dev. Biol. 117, 319-325. Wachtler, F. and Christ, B. (1992). The basic embryology of skeletal muscle formation in vertebrates: the avian model. Dev. Biol. 3, 217-227. Weintraub, H. (1993). The MyoD family and myogenesis: Redundancy, networks and thresholds. Cell 75, 1241-1244. Wolf, C., Thisse, C., Stoetzel, C., Thisse, B., Gerlinger, P., Perrin-Schmitt, F. (1991). The M-twist gene of Mus is expressed in subsets of mesodermal cells and is closely related to the Xenopus X-twi and the Drosophila twist genes. Dev. Biol. 143, 363-373. Yamada, T., Placzek, M. Tanaka, H., Dodd, J., and Jessell, T. M. (1991). Control of cell pattern in the developing nervous system: polarizing activity of the floor plate and notochord. Cell 64, 635-647. Yamada, T., Pfaff, S. L., Edlund, T., Jessell, T. M. (1993). Control of cell pattern in the neural tube: Motor neuron induction by diffusible factors from notochord and floor plate. Cell 73, 673-686. (Accepted 6 December 1994)