* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download SINTROM®
Survey
Document related concepts
Neuropharmacology wikipedia , lookup
Environmental impact of pharmaceuticals and personal care products wikipedia , lookup
Psychopharmacology wikipedia , lookup
Neuropsychopharmacology wikipedia , lookup
Prescription costs wikipedia , lookup
Pharmacognosy wikipedia , lookup
Psychedelic therapy wikipedia , lookup
Pharmacokinetics wikipedia , lookup
Drug interaction wikipedia , lookup
Adherence (medicine) wikipedia , lookup
Pharmacogenomics wikipedia , lookup
Theralizumab wikipedia , lookup
Discovery and development of direct thrombin inhibitors wikipedia , lookup
Transcript
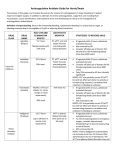
.2008 ידי משרד הבריאות ותוכנו נבדק ואושר על ידו באפריל-פורמט עלון זה נקבע על SINTROM® (acenocoumarol) 4 mg tablets Prescribing Information 1 Name of the medicinal product SINTROM® 2 Qualitative and quantitative composition Active ingredient: 3-[α-(4-nitrophenyl)-β-acetylethyl]-4-hydroxycoumarin (= acenocoumarol) as a racemic mixture. Acenocoumarol is a 4-hydroxycoumarin derivative. Tablets of 4 mg. 3 For a full list of excipients, see section 6.1 List of excipients.Pharmaceutical form Tablets for oral administration. SIN API APR08 CL V2 REF080806 Page 2 White, round, flat, with bevelled edges. One side bears the imprint "CG", the other is scored in the shape of a cross with the imprint "A" in each quadrant. 4 Clinical particulars 4.1 Therapeutic indications Anticuagulant derived from coumarin. 4.2 Posology and method of administration General guidelines Sensitivity to anticoagulants varies from patient to patient and may also fluctuate in the course of treatment. Therefore it is essential to perform regular testing of prothrombine time (PT)/ International Normalized Ratio (INR) and to adjust the patient's dosage accordingly. If this is not possible, Sintrom should not be used. The daily dosage should always be prescribed as a single dose and always taken at the same time of day. For adaptation of the dosage to various clinical conditions, see also section 4.4 Special warnings and precautions for use and section 4.5 Interaction with other medicinal products and other forms of interaction. Initial dosage The dosing of Sintrom must be individualized. The usual starting dose of Sintrom in a normal weight person is between 2 mg/day to 4 mg/day without administration of a loading dose, if the PT/INR value before the start of treatment is within the normal range. Treatment may also be initiated with a loading dose regimen, usually 6 mg on the first day followed by 4 mg on the second day. If the initial PT/INR value is abnormal, treatment should be instituted with caution. Elderly patients, patients with liver disease or severe heart failure with hepatic congestion or malnourished patients may require lower doses during treatment initiation and maintenance (see section 4.4 Special warnings and precautions for use). Before the start of treatment and up to the time when the coagulation status is stabilised within the therapeutic range, measurement of the PT/INR should be carried out on a daily basis. The interval between tests can later be extended, depending on the stability of PT/INR results. It is recommended that the blood samples for laboratory tests always be taken at the same time of day. Maintenance therapy and coagulation tests The maintenance dose varies from patient to patient and its appropriateness must be checked individually on the basis of PT/INR values. PT/INR should be assessed at regular intervals, i.e. at least once a month. The PT, which reflects the reduction of Vitamin K dependent clotting factors VII, X and II, is dependent on the responsiveness of the thromboplastin used for PT-testing. The responsiveness of the respective local thromboplastin compared to World Health SIN API APR08 CL V2 REF080806 Page 3 Organization international reference preparations is reflected by its International Sensitivity Index (ISI). The “International Normalised Ratio” (INR) was introduced for the purpose of standardization of the PT. The INR is the ratio of the patient's anticoagulated plasma PT to the normal plasma PT using the same thromboplastin in the same test system raised to the power of a value defined by the International Sensitivity Index. The maintenance dose generally lies between 1 and 8 mg daily depending on the individual patient, the underlying disease, clinical indication and the desired intensity of anticoagulation. Depending on the clinical indication, the optimal intensity of anticoagulation or therapeutic range to be aimed at generally lies between INR values of 2.0 and3.5(see table 1). Higher INR values up to 4.5 may be required in individual cases. Table 1 Indication Prophylaxis and treatment of venous thromboembolism (including pulmonary embolism) Atrial fibrillation Post-myocardial infarction (with increased risk for thromboembolic complications) Bioprosthetic heart valves Mechanical heart valves Recommended INR 2.0 – 3.0 2.0 – 3.0 2.0 – 3.0 2.0 – 3.0 2.0 – 3.5 Treatment with Sintrom can generally be discontinued without the need to taper off medication It has been found, however, that in extremely rare cases and in certain highrisk patients (e.g. after myocardial infarction) “rebound hypercoagulability” may occur . In such patients, withdrawal of anticoagulant therapy should be gradual. Missed Dose The anticoagulant effect of Sintrom persists beyond 24 hours. If the patient forgets to take the prescribed dose of Sintrom at the scheduled time, the dose should be taken as soon as possible on the same day. The patient should not take the missed dose by doubling the daily dose to make up for missed doses, but should refer back to his or her physician . Conversion from heparin therapy In clinical situations which require rapid anticoagulation, initial treatment with heparin is preferred since the anticoagulant effect of Sintrom is delayed. Conversion to Sintrom may begin concomitantly with heparin therapy or may be delayed depending on the clinical situation. To ensure continuous anticoagulation, it is advisable to continue full dose heparin therapy until Sintrom has produced the desired, stable therapeutic response as determined by PT/INR. During the transition phase close monitoring of anticoagulation is necessary. Treatment during dentistry and surgery Patients on Sintrom, who undergo surgical or invasive procedures require close surveillance of their coagulation status. Under certain conditions, e.g. when the operation site is limited and accessible to permit effective use of local procedures for hemostasis, SIN API APR08 CL V2 REF080806 Page 4 dental and minor surgical procedures may be performed during continued anticoagulation, without undue risk of hemorrhage. The decision to discontinue Sintrom, even for a short period of time, should carefully consider individual risks and benefits. The introduction of bridging anticoagulant treatment e.g. with heparin should be based on careful assessment of the expected risks of thromboembolism and bleeding. Use in children Experience with oral anticoagulants including acenocoumarol in children remains limited. Caution and more frequent monitoring of prothrombin time and INR is recommended. Use in elderly Elderly patients may require lower initial and maintenance doses .Elderly patients on anticoagulant therapy should be monitored with special care (see section 4.4 Special warnings and precautions for use and section 5.2 Pharmacokinetic properties). 4.3 • • • Contraindications Known hypersensitivity to acenocoumarol and related coumarin derivatives or to excipients. Pregnancy In patients unable to cooperate and who are unsupervised (e.g. unsupervised senile patients, alcoholics and patients with psychiatric disorders). Sintrom is also contraindicated in conditions where the risk of haemorrhage is greater than the possible clinical benefit, e.g.: • Haemorrhagic diathesis or haemorrhagic blood dyscrasia. • Shortly before or after surgical intervention on the central nervous system, as well as eye operations and traumatising surgery involving extensive exposure of tissues. • Peptic ulcers or haemorrhage in the gastrointestinal tract, urogenital tract, or respiratory system, as well as cerebrovascular haemorrhages, acute pericarditis and pericardial effusion, and infective endocarditis. • Severe hypertension, severe hepatic or renal disease. • Increased fibrinolytic activity as encountered after operations on the lung, prostate, uterus, etc. 4.4 Special warnings and precautions for use Strict medical supervision should be given in cases where the conditions or diseases may reduce the protein binding of Sintrom, for example, thyrotoxicosis, tumours, renal diseases, infections, and inflammation. Particular care should be taken in patients with hepatic dysfunction, since synthesis of coagulation factors may also be impaired or there may be an underlying platelet dysfunction (see also section 4.2 Posology and method of administration). Disorders affecting gastrointestinal absorption may alter the anticoagulant effect of Sintrom. In cases of severe heart failure, a very cautious dosage schedule must be adopted, because activation or gamma -carboxylation of the coagulation factors may be reduced in the SIN API APR08 CL V2 REF080806 Page 5 presence of hepatic congestion(see also section 4.2 Posology and method of administration). However with the reversal of hepatic congestion, it may be necessary to raise the dosage. Caution should be excercised in patients with known or suspected (e.g. abnormal bleeding after injury) protein C or protein S deficiency (see section 4.8 Undesirable effects). In elderly patients anticoagulant medication should be monitored with special care (see also section 4.2 Posology and method of administration). During treatment with anticoagulants, intramuscular injections may cause haematomas and should be avoided. Subcutaneous and intravenous injections, on the other hand, lead to no such complications. Meticulous care should be taken where it is necessary to shorten the PT/INR for diagnostic or therapeutic interventions (e.g. angiography, lumbar puncture, minor surgery, tooth extractions, etc.). Sintrom tablets contain lactose and are not recommended in patients with rare hereditary problems of galactose intolerance, of lactase deficiency or of glucose-galactose malabsorption. 4.5 Interaction with other medicinal products and other forms of interaction There are many possible interactions between coumarins and other drugs. The mechanisms of these interactions include disturbances of absorption, inhibition or induction of the metabolising enzyme system (mainly CYP2C9, see section 5.2 Pharmacokinetic properties), and reduced availability of the vitamin K necessary for the gamma -carboxylation of prothrombin-complex factors. It is important to note that some drugs may interact by more than one mechanism. Any form of therapy may involve the risk of an interaction although not all interactions will be significant. Thus careful surveillance is important and frequent (e.g. twice weekly) coagulation tests should be carried out when initially prescribing any drug in combination with Sintrom or withdrawing a concomitantly administered drug. 4.5.1 Effects of other drugs on acenocoumarolThe following drugs potentiate the anticoagulant activity of acenocoumarol and/or alter haemostasis and thereby increase the risk of haemorrhage: Heparin, platelet-aggregation inhibitors such as salicylic acid and its derivatives (e.g. acetylsalicylic acid, para-aminosalicylic acid, diflunisal), phenylbutazone or other pyrazolone derivatives (e.g. sulfinpyrazone), and other non-steroidal anti-inflammatory drugs including cyclo-oxygenase-2 inhibitors (e.g. celecoxib); high-dose intravenous methylprednisolone. Use of Sintrom together with these substances is therefore not recommended. When Sintrom is prescribed in combination with these drugs, coagulation tests should be performed more frequently. The following drugs may potentiate the anticoagulant effect of acenocoumarol: Allopurinol, anabolic steroids, androgens, antiarrhythmic agents (e.g. amiodarone, quinidine), antibiotics (e.g. amoxicillin, cephalosporins second and third generation, chloramphenicol, erythromycin, fluoroquinolones, neomycin, tetracyclines), cimetidine, disulfiram, ethacrynic acid, fibrates (e.g. clofibric acid), glucagon, imidazole derivatives (e.g. metronidazole and, even when administered locally, miconazole), paracetamol, SIN API APR08 CL V2 REF080806 Page 6 selective serotonin reuptake inhibitors SSRI (e.g. citalopram, fluoxetine, sertraline), statins (e.g. fluvastatin, atorvastatin, simvastatin), sulfonamides including co-trimoxazole (=sulfamethoxazole + trimethoprim), sulphonylureas (such as tolbutamide and chlorpropamide), thyroid hormones (incl. dextrothyroxine), tamoxifen and tramadol. Corticosteroids (e.g. methylprednisolone, prednisone). Corticosteroids have also been reported to diminish the anticoagulant effect of coumarin derivatives. Inhibitors of CYP2C9 may potentiate the anticoagulant effect of acenocoumarol. The following drugs may diminish the anticoagulant effect of acenocoumarol: Aminoglutethimide, antineoplastic drugs (azathioprine, 6-mercaptopurine), barbiturates (e.g. phenobarbital), carbamazepine, cholestyramine (see section 4.9 Overdose), HIV protease inhibitors (e.g. ritonavir, nelfinavir), griseofulvin, oral contraceptives, rifampicin and St. John’s wort / hypericum perforatum (this interaction has been described with warfarin and phenprocoumon and can not be ruled out for acenocoumarol). Inducers of CYP2C9, CYP2C19 or CYP3A4 may diminish the anticoagulant effect of acenocoumarol. Since neither the severity nor the early signs of interactions can be predicted, patients taking Sintrom, especially if they also suffer from hepatic dysfunction, should limit their alcohol intake. 4.5.2 Effects of acenocoumarol on other drugs During concomitant treatment with hydantoin derivatives(such as phenytoin), the serum hydantoin concentration may rise. Sintrom may potentiate the hypoglycaemic effect of sulfonylurea derivatives. 4.6 Pregnancy and lactation Pregnancy Sintrom, like other coumarin derivatives, may be associated with congenital malformation of the embryo. Sintrom is therefore contraindicated during pregnancy. Women of child-bearing potential Women of childbearing potential should take contraceptive measures during treatment with Sintrom. Lactation Sintrom passes into the breast milk of lactating mothers, but, with limited data available, the quantities in breast milk are small and undesirable effects on the infant are usually not to be expected. The decision to breast-feed should be carefully considered and may include coagulation tests and vitamin K status evaluation in infants before advising women to breast-feed. Women who are breast-feeding and treated with Sintrom should be carefully monitored to ensure that recommended PT/INR values are not exceeded. When breast-feeding, the infant should be given 1 mg vitamin K1 per week as a prophylactic. SIN API APR08 CL V2 REF080806 Page 7 4.7 Effects on ability to drive and use machines Sintrom has no known influence on the ability to drive or use machines. Out-patients should nevertheless be advised to carry with them an 'anticoagulant card' in view of the possibility of their sustaining injuries. 4.8 Undesirable effects Adverse reactions are ranked under headings of frequency, the most frequent first, using the following convention: Very common (≥ 1/10); common (≥ 1/100, < 1/10); uncommon (≥ 1/1000, < 1/100); rare (≥ 1/10,000, < 1/1000); very rare (< 1/10,000), including isolated reports. Haemorrhage Haemorrhage in various organs is a common side effect associated with Sintrom, its occurrence is related to the dosage to the drug, the patient’s age, and the nature of the underlying disease (but not to the duration of treatment). Table 2 Immune system disorders Rare: Allergic reactions (e.g. urticaria, rash) Vascular disorders Very rare: Vasculitis Gastrointestinal disorders Rare: Loss of appetite, nausea, vomiting Hepatobiliary disorders Very rare: Liver damage Skin and subcutaneous tissue disorders Rare: Alopecia Very rare: Skin necrosis haemorrhagic (usually associated with congenital deficiency of protein C or its cofactor protein S) 4.9 Overdose Whereas single doses even if very large do not usually prove dangerous, clinical manifestations of overdosage may set in during prolonged use of daily doses higher than are necessary for treatment. Signs and symptoms The onset and severity of the symptoms are dependent on the individual's sensitivity to oral anticoagulants, the severity of the overdosage, and the duration of treatment. Bleeding is the major sign of poisoning with oral anticoagulant drugs. The most frequent symptoms observed are: cutaneous bleeding (80%), haematuria (52%), haematomas, gastrointestinal bleeding, haematemesis, uterine bleeding, epistaxis, gingival bleeding and bleeding into the joints. Laboratory tests reveal an extremely high PT/INR value, pronounced prolongation of the recalcification time or thromboplastin time, and disturbed gamma-carboxylation of factors II, VII, IX, and X. SIN API APR08 CL V2 REF080806 Page 8 Treatment The necessity, or desirability, of the treatment with syrup ipecac, gastric lavage in addition to the activated charcoal and cholestyramine administration is controversial. The benefits of these treatments should be balanced against the risk of bleeding in each patient. If the patient has not been previously receiving anticoagulants, presents for treatment within 1 hour of ingestion, is not obtunded, comatose, or convulsing, and has no evidence of bleeding from any source, emesis with syrup of ipecac and gastric lavage with a largebore orogastric tube can be attempted. Gastric lavage may also provoke bleeding. After gastric lavage, activated charcoal may be administered. In patients who are already anticoagulated, emesis should not be induced. Vitamin-K mediated reversal of anticoagulation may be dangerous for patients who require constant anticoagulations (e.g. for prostetic heart valves). Cholestyramine may markedly increase elimination of the drug by inhibiting the enterohepatic circulation. Emergency and supportive measures: In emergency situations of severe haemorrhage, clotting factors can be returned to normal by administering fresh whole blood or fresh frozen plasma, complex concentrate or recombinant factor VIIa supplemented with vitamin K1. Antidote Vitamin K1- (phytomenadione) may antagonise the inhibitory effect of Sintrom on hepatic gamma-carboxylation of the vitamin K-dependent coagulation factors within 3-5 hours. In the event of clinically insignificant haemorrhages, such as brief nose-bleed or small isolated haematomas, a temporary reduction of the dose of Sintrom is often sufficient. In cases of moderate haemorrhage, give 2-5 mg vitamin K1 by oral route. If there is evidence of significant anticoagulation give 5-10 mg vitamin K1 very slowly i.v. (at a rate not exceeding 1 mg per minute). 5 Pharmacological properties 5.1 Pharmacodynamic properties Pharmacotherapeutic group: Antithrombotic, vitamin K antagonists. ATC code: B01AA07 Acenocoumarol, the active substance of Sintrom, is a coumarin derivative and functions as vitamin K antagonist. Vitamin K antagonists produce their anticoagulant effect by inhibition of the vitamin K-epoxide-reductase with a subsequent reduction of the gammacarboxylation of certain glutamic acid molecules which are located at several sites near the terminal end both of coagulation factors II (prothrombin), VII, IX, and X, and of protein C or its cofactor protein S. This gamma-carboxylation has a significant bearing on interaction of the aforementioned coagulation factors with Ca ions. Without this reaction, blood clotting cannot be initiated. Depending on the size of the initial dosage, acenocoumarol causes prolongation of the PT/INR within approx. 36 - 72 hours. Following withdrawal of the medication the PT/INR usually reverts to normal after a few days. SIN API APR08 CL V2 REF080806 Page 9 5.2 Pharmacokinetic properties Absorption Acenocoumarol, a racemic mixture of the optical R(+) and S(-) enantiomers, is rapidly absorbed by the oral route, and at least 60% of the dose is systemically available. Peak plasma concentrations of 0.3 ± 0.05 microgram/mL are attained within 1-3 hours after a single dose of 10 mg. The peak plasma concentrations and the areas under the blood concentration curve (AUC) are proportional to the dose over a dosage range of 8-16 mg. The between-patient plasma concentrations vary to such an extent that no correlation can be established between the dose, plasma concentrations of acenocoumarol and the apparent prothrombin level. Distribution The bulk of the acenocoumarol administered is to be distributed in the plasma fraction of the blood, where 98.7% becomes bound to plasma proteins, notably to albumin. The apparent volume of distribution is 0.16-0.18 L/kg for the R(+) enantiomer and 0.22-0.34 L/kg for the S(-) enantiomer. Acenocoumarol passes into the breast milk, but only in very small quantities which cannot be detected by the usual analytical methods. It also crosses the placental barrier. BiotransformationAcenocoumarol is extensively metabolised. 6- and 7-hydroxylation of both enantiomers of acenocoumarol are the major metabolites and the cytochrome P450 2C9 is the major catalyst for the formation of these four metabolites. Other enzymes involved in the metabolism of (R)-acenocoumarol are CYP1A2 and CYP2C19.By reduction of the keto group two different carbinol metabolites are formed. Reduction of the nitro group results in an amino metabolite. None of these metabolites contribute to the anticoagulant activity of the parent drug in man, but they are all active in an animal model. CYP2C9-related genetic variability accounts for 14% of the interindividual variability in acenocoumarol pharmacodynamic response. Elimination Acenocoumarol is eliminated from the plasma with a half-life of 8-11 hours. The apparent plasma clearance amounts to 3.65 L/h after oral administration. The total plasma clearance of the R (+) enantiomer of acenocoumarol, which possesses significantly higher anticoagulant activity, is much lower than that of the S(-) enantiomer. Only 0.12-0.18% of the dose is excreted unchanged in the urine. Cumulative excretion of metabolites and acenocoumarol over 1 week amounts to 60% of the dose in urine and 29% in the faeces. Characteristics in patients In one study, the plasma concentrations of acenocoumarol which produced a given prothrombin level appeared to be higher in patients over 70 years of age than in younger patients although the doses administered were not larger. SIN API APR08 CL V2 REF080806 Page 10 5.3 Preclinical safety data Toxicity After a single (acute) oral and/or intravenous dose, acenocoumarol showed a low degree of toxicity in mice, rats, and rabbits. The dog showed moderate toxicity. In repeated-dose studies, the liver is suggested to be the main target organ in the toxicity of coumarin derivatives including acenocoumarol. The administration of these substances at excessive pharmacological doses can cause haemorrhages. Reproduction toxicity, teratogenicity No animal experiments were performed with acenocoumarol. However, placental and transplacental interference with vitamin K dependent coagulation factors may give rise to embryonic or fetal anomalies and neonatal haemorrhages both in animals and in humans. Mutagenicity From investigations on bacterial and mammalian cell systems in vitro, including a DNA repair assay on rat hepatocytes, it can be concluded that acenocoumarol and/or its metabolites did not exert any mutagenic effects. An in vitro study on human lymphocytes has shown some mild mutagenic activity. However, in this experiment the effective concentrations of acenocoumarol, ≥188 and ≥250 microgram/mL (with and without metabolic activation, respectively), were 500 to 1000 times higher than concentrations determined in human plasma after medication with acenocoumarol. Carcinogenicity No lifetime-exposure studies were carried out in animals with acenocoumarol. Coumarin, at doses clearly exceeding the maximum tolerated dose (MTD), induced an increase in the incidence of liver tumours in rats, without impact on survival. No such finding was recorded for mice. The induction of hepatoma seen in rats with anticoagulants of the coumarin class is not likely to indicate an increased carcinogenic risk in humans. Hepatoxicity of coumarin and its derivatives in the rat is understood to be associated with enzyme induction and the metabolic pathway of coumarin and/or its metabolites peculiar to this rodent species. 6 Pharmaceutical particulars 6.1 List of excipients Anhydrous colloidal silica (silica aerogel), lactose monohydrate, magnesium stearate, maize starch and pregelatinized maize starch. 6.2 Special precautions for storage Sintrom tablets do not require any special storage conditions. Sintrom must be kept out of the reach and sight of children. Manufacturer: SIN API APR08 CL V2 REF080806 Page 11 Novartis Farma SPA, Torre Annunziata, Italy For: Novartis Pharma AG, Basel, Switzerland. Lisence holder: PharmaExcel Ltd. 23 Hasivim St. Petach-Tikva SIN API APR08 CL V2 REF080806