* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download The importance of exercise echocardiography for clinical decision
Heart failure wikipedia , lookup
Remote ischemic conditioning wikipedia , lookup
Cardiac contractility modulation wikipedia , lookup
Management of acute coronary syndrome wikipedia , lookup
Coronary artery disease wikipedia , lookup
Cardiothoracic surgery wikipedia , lookup
Rheumatic fever wikipedia , lookup
Artificial heart valve wikipedia , lookup
Arrhythmogenic right ventricular dysplasia wikipedia , lookup
Atrial fibrillation wikipedia , lookup
Quantium Medical Cardiac Output wikipedia , lookup
Hypertrophic cardiomyopathy wikipedia , lookup
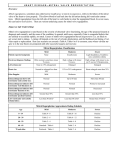
Systematic review The importance of exercise echocardiography for clinical decision making in primary mitral regurgitation Caroline M. Van de Heyning, Julien Magne, Patrizio Lancellotti and Luc A. Piérard Primary mitral regurgitation is generally an insidious disease with late onset of symptoms. Current European and American guidelines recommend surgery in severe primary mitral regurgitation when symptoms, overt left ventricular dysfunction, pulmonary hypertension or atrial fibrillation, occur. However, recent large studies reported an improved outcome in asymptomatic patients with severe mitral regurgitation referred for early mitral valve repair despite the risk of operative mortality or mitral valve replacement. Moreover, primary mitral regurgitation appears to have an important dynamic character in up to one-third of patients. This article provides an overview of the incremental evidence of the ability of exercise echocardiography to Introduction Mitral regurgitation is the second most prevalent valvulopathy in Europe after aortic stenosis.1 Primary mitral regurgitation has an organic cause such as rheumatic disease, myxomatous degeneration or endocarditis. In secondary mitral regurgitation, the mitral valve is structurally normal but dysfunctional due to ischaemic or non-ischaemic cardiomyopathy. The disease course of primary mitral regurgitation is usually insidious due to progressive atrial and left ventricular remodelling which compensates for the overload caused by the increasing regurgitant volume.2 Although the late onset of symptoms often leads to late referral for surgery,3 the outcome of medically treated asymptomatic severe primary mitral regurgitation remains unclear. Enriquez-Sarano et al.4 reported a 5-year probability of 62 þ 8% for cardiac death, heart failure or new atrial fibrillation in asymptomatic patients with severe mitral regurgitation, whereas more recently Kang et al.5 found a 7-year survival free of cardiac death or heart failure of 85 þ 4% in asymptomatic mitral regurgitation with a preserved left ventricular ejection fraction (LVEF). The 2007 European Society of Cardiology (ESC) and 2008 American College of Cardiology (ACC)/America Heart Association (AHA) guidelines recommend surgery in severe primary mitral regurgitation with symptoms including LVEF of 60% or less, left ventricular dilatation, atrial fibrillation and/or pulmonary hypertension (PHT) (Table 1).6,7 Rosenhek et al.8 showed an excellent outcome in asymptomatic severe mitral regurgitation with the ‘watchful waiting’ strategy in accordance with these guidelines. Nevertheless, numerous large studies have provided evidence for a 1558-2027 ß 2012 Italian Federation of Cardiology assess the functional repercussions of mitral regurgitation and the identification of high-risk patients who might benefit from early referral for surgery. J Cardiovasc Med 2012, 13:260–265 Keywords: exercise echocardiography, primary mitral regurgitation University of Liege, University Hospital Sart Tilman, Liege, Belgium Correspondence to Luc A. Piérard, MD, PhD, University Hospital Sart Tilman, Liege 400, Belgium Tel: +32 4 366 8870; fax: +32 4 366 7195; e-mail: [email protected] Received 19 October 2011 Revised 8 November 2011 Accepted 29 November 2011 more favourable clinical course if patients are submitted to surgery before left ventricular dysfunction or PHT has developed.9–12 It is well established that mitral valve repair yields a better prognosis than mitral valve replacement regarding operative mortality, postoperative LVEF and prosthesis-related complications.2,3 Despite a low operative mortality risk of 0.6% for asymptomatic patients and increasing repairability rates,13 it should be noted that there is a considerable variability in the frequency of mitral valve repair between high and low volume centres and surgeons.3,14 As a result, a heated debate persists about the optimal timing for surgery.15–17 Moreover degenerative mitral regurgitation appears to be a dynamic disease with significant progression of mitral regurgitation during exercise in one-third of patients, which is associated with a worse outcome.18,19 Risk stratification to identify patients who could benefit from early surgery appears to be crucial in the current management of primary mitral regurgitation. In this setting, several recent articles have pointed out the emerging role of exercise echocardiography.20–23 The most recent guidelines of the European Association of Echocardiography (EAE)24 only recommend exercise echocardiography in primary mitral regurgitation when symptoms are discordant with mitral regurgitation severity at rest. This article will give an overview of the current utility of exercise echocardiography in clinical decision making. Exercise-induced haemodynamic changes in primary mitral regurgitation The dynamic character of secondary mitral regurgitation and its implications are well established.25 Conversely, DOI:10.2459/JCM.0b013e3283515c70 Copyright © Italian Federation of Cardiology. Unauthorized reproduction of this article is prohibited. Exercise echocardiography in primary MR Van de Heyning et al. 261 Surgical criteria for mitral valve repair in severe primary mitral regurgitation Table 1 Surgical criterion Symptomatic severe MR LVEF < 60% End-systolic diameter > 45 mm End-systolic diameter > 40 mm SPAP > 50 mmHg at rest SPAP > 60 mmHg at exercise Atrial fibrillation High probability mitral valve repair, low comorbidity Probability mitral valve repair >90%, expert centre 2007 ESC guidelines7 2008 ACC/AHA guidelines6 Class I Class I Class I Class I Class I Class IIa Class IIa Class IIb Class Class Class Class I IIa IIa IIa Class IIa ACC, American College of Cardiology; AHA, American Heart Association; ESC, European Society of Cardiology; LVEF, left ventricular ejection fraction; MR, mitral regurgitation; SPAP, systolic pulmonary arterial pressure. few studies exist about exercise-induced changes in primary mitral regurgitation. Stoddard et al.18 showed that 32% of patients with mitral valve prolapse without mitral regurgitation developed mitral regurgitation during exercise. More recently and in line with these data, Magne et al.19 found an increase in mitral regurgitation (regurgitant volume > 15 ml or effective regurgitant orifice area > 10 mm2) in one-third of asymptomatic patients with moderate-to-severe degenerative mitral regurgitation. Interestingly, according to the effective regurgitant orifice area, 32% of patients with moderate mitral regurgitation at rest developed severe mitral regurgitation at exercise, whereas, respectively, 15 and 7% of patients with severe and moderate mitral regurgitation dropped one class. Both studies observed in these patients an increased mitral valve annulus area which could point at exercise-induced progressive ring dilatation with suboptimal leaflet coaptation as a possible mechanism. The smaller cavity during exercise with subsequent less tugging of the chordae and, thus, worsening mitral valve prolapse might be an additional factor in degenerative mitral regurgitation.19 The rise in blood pressure during exercise did not seem to play a major role in increasing mitral regurgitation in the latter study. Importantly, there was a strong correlation between exercise-induced mitral regurgitation progression and exercise PHT. in symptomatic patients with less-than-severe mitral regurgitation in order to assess the haemodynamic consequences of mitral regurgitation. Continuous echocardiographic examination during semisupine ergometry is preferred over postexercise echocardiography, as measurements can differ significantly between peak stress and the immediate postexercise period,22 specifically regarding the SPAP. After the patient is positioned on the ergometer, a workload of 50 W is set. During the test, the workload is increased with 25 W every 3 min in young, otherwise healthy patients, but can be adjusted in older individuals. Heart rate, blood pressure and symptoms should be noted at each level and during recovery, as well as exercise duration (Fig. 1). Meanwhile, image acquisition is performed with special attention to the systolic left ventricular function, the evolution of mitral regurgitation severity and the SPAP. Data from expert echo laboratories show a high feasibility and reproducibility of mitral regurgitation quantification and a good agreement between the flow convergence method and the Doppler volumetric method both at rest and during exercise,19,28 although this assessment might be challenging in less experienced hands.23 Exercise echocardiography is not recommended in severe mitral regurgitation with symptoms or a LVEF less than 60% (indications for surgery), severe hypertension, important arrhythmias, systemic illness or the impossibility to perform an adequate exercise test.22 Assessment of functional repercussions of mitral regurgitation As in non-valvular cardiac abnormality,29 diastolic dysfunction appears to be a major determinant in reduced functional capacity in primary mitral regurgitation, Fig. 1 How to perform exercise echocardiography in primary mitral regurgitation First, the patients’ medical history and a directed physical examination should be obtained to identify symptoms. Second, a rest echocardiography should be performed according to the guidelines26,27 to identify and localize the valvular lesion and to quantify mitral regurgitation severity, LVEF, left ventricular dimensions (especially the left ventricular end-systolic diameter) and the systolic pulmonary arterial pressure (SPAP). Subsequently, exercise echocardiography is recommended in asymptomatic patients with severe mitral regurgitation or Setting semisupine ergometer in exercise echocardiography. Echocardiographic images are acquired at rest, during several steps of exercise and during recovery. Meanwhile, continuous 12-lead ECG recording is performed and blood pressure and symptoms are noted at each step. Copyright © Italian Federation of Cardiology. Unauthorized reproduction of this article is prohibited. 262 Journal of Cardiovascular Medicine 2012, Vol 13 No 4 regardless of compensatory left atrial dilatation.30,31 Moreover, resting left ventricular filling pressures may predict exercise PHT32 which is correlated with a decreased exercise capacity.33 To a lesser extent, reduction of exercise tolerance is associated with a lower exercise cardiac output,31 caused by the inability of the left ventricle to maintain a large forward stroke volume. Exercise forward stroke volume in mitral regurgitation is determined by two opposing mechanisms, namely contractile reserve and eventually increasing mitral regurgitation.23 The presence of contractile reserve itself is associated with a significantly better functional capacity in comparison with lack of contractile reserve.34 Fig. 2 Due to the slow disease progression, the patient may be unaware of their adaptation of daily activities and will often deny symptoms. It is well recognized that there is a primary role for exercise testing to unmask these ‘asymptomatic’ patients.35 Current EAE guidelines advocate exercise echocardiography when symptoms are discrepant with mitral regurgitation severity at rest.24,26 These recommendations are based on a small study by Tischler et al.36 which found that exertional dyspnoea in mild rheumatic valve disease is frequently explained by an increase in mitral regurgitation combined with exercise PHT. This observation was recently confirmed by a study in 78 asymptomatic patients with moderate-to-severe degenerative mitral regurgitation in which patients with exercise-induced increase in mitral regurgitation had higher SPAP, more frequent exercise PHT and presented more often dyspnoea during exercise33 (Fig. 2). In brief, there is incremental evidence that exercise echocardiography is an interesting tool for the objective evaluation of the functional repercussions of mitral regurgitation. Identification of high-risk patients There is not yet a consensus about the optimal timing for surgery in asymptomatic patients with severe mitral regurgitation and preserved left ventricular systolic function. Although a LVEF less than 60% is highly predictive for postoperative left ventricular deterioration,9 a decreased LVEF is only a late marker of left ventricular dysfunction in significant primary mitral regurgitation. Indeed, in these patients, the LVEF remains generally normal or supranormal due to the increased preload and the alternative low impedance pathway provided by the regurgitant orifice.37,38 Detection of subclinical systolic left ventricular dysfunction in patients with severe mitral regurgitation is, therefore, mandatory to avoid postoperative overt left ventricular impairment. In recent years, exercise echocardiography has become an important tool for this purpose.39 The absence of contractile reserve, initially defined as less than 4% increase in the LVEF at exercise, predicts a worse postoperative LVEF and event-free survival in minimally symptomatic severe mitral regurgitation patients submitted to surgery.34,40 Example of an exercise echocardiogram in a 78-year-old male patient with severe primary mitral regurgitation and a normal ejection fraction of 67%. The first column represents images at rest of, respectively, the colour flow mapping of the mitral regurgitation, the velocity–time integral of the regurgitant jet and the transtricuspid gradient (both measured by continuous Doppler). The second column depicts the same images during exercise and shows a slight increase in the effective regurgitant orifice (from 40 to 46 mm2) and the regurgitant volume (from 63 to 72 ml) with concomitant increase of the systolic pulmonary arterial pressure (increase of the transtricuspid gradient from 55 to 74 mmHg). Alternatively, contractile reserve can be assessed by two-dimensional speckle-tracking whereby less than 1.9% increase in global longitudinal strain (GLS) at peak exercise predicts postoperative left ventricular deterioration in asymptomatic patients41 (Fig. 3). Recent data from our echocardiography laboratory show that evaluation of contractile reserve by GLS is more accurate than by ejection fraction in predicting adverse outcome (accepted abstract ESC Congress 2011). Contractile reserve can also be appreciated by pharmacological stress testing, its only indication in primary mitral regurgitation.24 Apart from revealing early left ventricular dysfunction, exercise echocardiography is also useful for identification of several risk factors which yield a poor outcome. First, it has been shown that decreased exercise capacity itself, marked by a reduced peak oxygen consumption during cardiopulmonary exercise testing31 or a diminished duration at exercise tolerance test,42 is predictive for mortality and the development of symptoms or other criteria for surgery in asymptomatic mitral regurgitation. Second, both exercise PHT (>60 mmHg)33 and exerciseinduced increase in mitral regurgitation severity19 have recently been shown to be strongly correlated with a Copyright © Italian Federation of Cardiology. Unauthorized reproduction of this article is prohibited. Exercise echocardiography in primary MR Van de Heyning et al. 263 Fig. 3 Absence of contractile reserve in a patient with severe primary mitral regurgitation. The upper panel represents two-dimensional strain calculations at rest for the patient also used for Fig. 2. The lower figure shows the two-dimensional strain values during exercise. Despite an increase in mitral regurgitation and ejection fraction, there was almost no increase in global longitudinal strain (23.7% at rest versus 24.0% during exercise), indicating subclinical left ventricular dysfunction. Copyright © Italian Federation of Cardiology. Unauthorized reproduction of this article is prohibited. 264 Journal of Cardiovascular Medicine 2012, Vol 13 No 4 decreased symptom-free survival. On the basis of these data, the 2010 EAE recommendations26 state that the absence of contractile reserve, a limited exercise capacity, the presence of exercise PHT or an exercise-induced increase in mitral regurgitation severity may identify high-risk patients who might benefit from early surgery. At least a closer follow-up should be provided in these high-risk patients, as in patients who almost fulfil the surgical criteria. The 2008 ACC/AHA guidelines6 already adopted exercise PHT more than 60 mmHg as a class IIa indication for surgery. However, it should be noted that over one-third of normal individuals older than 60 years present PHT of at least 60 mmHg at peak exercise,43 and that exercise PHT can be predicted by age and rest echocardiographic parameters.32 Conclusion In patients with primary mitral regurgitation, current guidelines support the use of exercise echocardiography in patients with discordant symptoms, to evaluate the functional repercussion of mitral regurgitation in an objective manner. Like secondary mitral regurgitation, primary mitral regurgitation appears to be a dynamic condition which is incompletely characterized at rest. Recent studies show that limited exercise capacity and exercise-induced mitral regurgitation progression or exercise PHT are correlated with the development of symptoms. Furthermore, there is growing evidence that exercise echocardiography should be performed on a regular basis in asymptomatic patients with severe mitral regurgitation to detect absence of contractile reserve which points at subclinical left ventricular dysfunction. Implementation of exercise echocardiography in asymptomatic severe primary mitral regurgitation permits a more precise identification of patients who require a closer follow-up and who might benefit from early referral for surgery. Watchful waiting seems more appropriate in patients without the above-mentioned risk factors or when mitral valve repair is unlikely. References 1 2 3 4 5 6 Iung B, Baron G, Butchart EG, et al. A prospective survey of patients with valvular heart disease in Europe: the Euro Heart Survey on valvular heart disease. Eur Heart J 2003; 24:1231–1243. Enriquez-Sarano M, Akins CW, Vahanian A. Mitral regurgitation. Lancet 2009; 373:1382–1394. Anyanwu AC, Bridgewater B, Adams DH. The lottery of mitral valve repair surgery. Heart 2010; 96:1964–1967. Enriquez-Sarano M, Avierinos JF, Messika-Zeitoun D, et al. Quantitative determinants of the outcome of asymptomatic mitral regurgitation. N Engl J Med 2005; 352:875–883. Kang DH, Kim JH, Rim JH, et al. Comparison of early surgery versus conventional treatment in asymptomatic severe mitral regurgitation. Circulation 2009; 119:797–804. Bonow RO, Carabello BA, Chatterjee K, et al. 2008 focused update incorporated into the ACC/AHA 2006 guidelines for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to revise the 1998 guidelines for the management of patients with valvular heart disease). Endorsed by the Society of Cardiovascular Anesthesiologists, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons. J Am Coll Cardiol 2008; 52:e1–e142. 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 26 27 28 29 Vahanian A, Baumgartner H, Bax J, et al. Guidelines on the management of valvular heart disease: the Task Force on the Management of Valvular Heart Disease of the European Society of Cardiology. Eur Heart J 2007; 28:230–268. Rosenhek R, Rader F, Klaar U, et al. Outcome of watchful waiting in asymptomatic severe mitral regurgitation. Circulation 2006; 113:2238– 2244. Enriquez-Sarano M, Tajik AJ, Schaff HV, Orszulak TA, Bailey KR, Frye RL. Echocardiographic prediction of survival after surgical correction of organic mitral regurgitation. Circulation 1994; 90:830–837. Tribouilloy C, Grigioni F, Avierinos JF, et al. Survival implication of left ventricular end-systolic diameter in mitral regurgitation due to flail leaflets a long-term follow-up multicenter study. J Am Coll Cardiol 2009; 54:1961– 1968. Barbieri A, Bursi F, Grigioni F, et al. Prognostic and therapeutic implications of pulmonary hypertension complicating degenerative mitral regurgitation due to flail leaflet: a Multicenter Long-term International Study (on behalf of the Mitral Regurgitation International DAtabase (MIDA) Investigators). Eur Heart J 2011; 32:751–759. Le Tourneau T, Richardson M, Juthier F, et al. Echocardiography predictors and prognostic value of pulmonary artery systolic pressure in chronic organic mitral regurgitation. Heart 2010; 96:1311–1317. Gammie JS, Sheng S, Griffith BP, et al. Trends in mitral valve surgery in the United States: results from the Society of Thoracic Surgeons Adult Cardiac Surgery Database. Ann Thorac Surg 2009; 87:1431–1437. Bolling SF, Li S, O’Brien SM, Brennan JM, Prager RL, Gammie JS. Predictors of mitral valve repair: clinical and surgeon factors. Ann Thorac Surg 2010; 90:1904–1911. Enriquez-Sarano M, Sundt TM. Early surgery is recommended for mitral regurgitation. Circulation 2010; 121:804–811. Gillam LD, Schwartz A. Primum non nocere: the case for watchful waiting in asymptomatic ‘severe’ degenerative mitral regurgitation. Circulation 2010; 121:813–821. Suri RM, Avierinos JF, Dearani JA, et al. Management of less-than-severe mitral regurgitation: should guidelines recommend earlier surgical intervention? Eur J Cardiothorac Surg 2011; 40:496–502. Stoddard MF, Prince CR, Dillon S, Longaker RA, Morris GT, Liddell NE. Exercise-induced mitral regurgitation is a predictor of morbid events in subjects with mitral valve prolapse. J Am Coll Cardiol 1995; 25:693–699. Magne J, Lancellotti P, Piérard LA. Exercise-induced changes in degenerative mitral regurgitation. J Am Coll Cardiol 2010; 56:300–309. Picano E, Pibarot P, Lancellotti P, Monin JL, Bonow RO. The emerging role of exercise testing and stress echocardiography in valvular heart disease. J Am Coll Cardiol 2009; 54:2251–2260. Yared K, Lam KM, Hung J. The use of exercise echocardiography in the evaluation of mitral regurgitation. Curr Cardiol Rev 2009; 5:312– 322. O’Connor K, Lancellotti P, Piérard LA. Stress Doppler echocardiography in valvular heart diseases: utility and assessment. Future Cardiol 2010; 6:611–625. Flachskampf FA. Mitral regurgitation is incompletely characterized at rest. J Am Coll Cardiol 2010; 56:310–313. Sicari R, Nihoyannopoulos P, Evangelista A, et al. Stress echocardiography expert consensus statement: European Association of Echocardiography (EAE) (a registered branch of the ESC). Eur J Echocardiogr 2008; 9:415– 437. Lancellotti P, Gérard PL, Piérard LA. Long-term outcome of patients with heart failure and dynamic functional mitral regurgitation. Eur Heart J 2005; 26:1528–1532. Lancellotti P, Moura L, Pierard LA, et al. European Association of Echocardiography recommendations for the assessment of valvular regurgitation. Part 2: Mitral and tricuspid regurgitation (native valve disease). Eur J Echocardiogr 2010; 11:307–332. Zoghbi WA, Enriquez-Sarano M, Foster E, et al. Recommendations for evaluation of the severity of native valvular regurgitation with twodimensional and Doppler echocardiography (American Society of Echocardiography). J Am Soc Echocardiogr 2003; 16:777–802. Enriquez-Sarano M, Miller FA, Hayes SN, Bailey KR, Tajik AJ, Seward JB. Effective mitral regurgitant orifice area: clinical use and pitfalls of the proximal isovelocity surface area method. J Am Coll Cardiol 1995; 25:703–709. Montant P, Chenot F, Robert A, et al. Long-term survival in asymptomatic patients with severe degenerative mitral regurgitation: a propensity score-based comparison between an early surgical strategy and a conservative treatment approach. J Thorac Cardiovasc Surg 2009; 138:1339–1348. Copyright © Italian Federation of Cardiology. Unauthorized reproduction of this article is prohibited. Exercise echocardiography in primary MR Van de Heyning et al. 265 30 31 32 33 34 35 36 Kim HK, Kim YJ, Chung JW, Sohn DW, Park YB, Choi YS. Impact of left ventricular diastolic function on exercise capacity in patients with chronic mitral regurgitation: an exercise echocardiography study. Clin Cardiol 2004; 27:624–628. Messika-Zeitoun D, Johnson BD, Nkomo V, et al. Cardiopulmonary exercise testing determination of functional capacity in mitral regurgitation: physiologic and outcome implications. J Am Coll Cardiol 2006; 47:2521–2527. Magne J, Lancellotti P, O’Connor K, Van de Heyning CM, Szymanski C, Piérard LA. Prediction of exercise pulmonary hypertension in asymptomatic degenerative mitral regurgitation. J Am Soc Echocardiogr 2011; 24:1004–1012. Magne J, Lancellotti P, Piérard LA. Exercise pulmonary hypertension in asymptomatic degenerative mitral regurgitation. Circulation 2010; 122:33–41. Lee R, Haluska B, Leung DY, Case C, Mundy J, Marwick TH. Functional and prognostic implications of left ventricular contractile reserve in patients with asymptomatic severe mitral regurgitation. Heart 2005; 91:1407–1412. Piérard LA, Lancellotti P. Stress testing in valve disease. Heart 2007; 93:766–772. Tischler MD, Battle RW, Saha M, Niggel J, LeWinter MM. Observations suggesting a high incidence of exercise-induced severe mitral regurgitation in patients with mild rheumatic mitral valve disease at rest. J Am Coll Cardiol 1995; 25:128–133. 37 38 39 40 41 42 43 de Isla LP, De Agustin A, Rodrigo JL, et al. Chronic mitral regurgitation: a pilot study to assess preoperative left ventricular contractile function using speckle-tracking echocardiography. J Am Soc Echocardiogr 2009; 22:831–838. Hetzer R, Dandel M. Early detection of left ventricular dysfunction in patients with mitral regurgitation due to flail leaflet is still a challenge. Eur Heart J 2011; 32:665–667. Lee R, Marwick TH. Assessment of subclinical left ventricular dysfunction in asymptomatic mitral regurgitation. Eur J Echocardiogr 2007; 8:175–184. Leung DY, Griffin BP, Stewart WJ, Cosgrove DM, Thomas JD, Marwick TH. Left ventricular function after valve repair for chronic mitral regurgitation: predictive value of preoperative assessment of contractile reserve by exercise echocardiography. J Am Coll Cardiol 1996; 28:1198–1205. Lancellotti P, Cosyns B, Zacharakis D, et al. Importance of left ventricular longitudinal function and functional reserve in patients with degenerative mitral regurgitation: assessment by two-dimensional speckle tracking. J Am Soc Echocardiogr 2008; 21:1331–1336. Supino PG, Borer JS, Schuleri K, et al. Prognostic value of exercise tolerance testing in asymptomatic chronic nonischemic mitral regurgitation. Am J Cardiol 2007; 100:1274–1281. Mahjoub H, Levy F, Cassol M, et al. Effects of age on pulmonary artery systolic pressure at rest and during exercise in normal adults. Eur J Echocardiogr 2010; 10:635–640. Copyright © Italian Federation of Cardiology. Unauthorized reproduction of this article is prohibited.