* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Capillary Electrophoresis System
List of types of proteins wikipedia , lookup
Protein (nutrient) wikipedia , lookup
Gel electrophoresis of nucleic acids wikipedia , lookup
Protein moonlighting wikipedia , lookup
Agarose gel electrophoresis wikipedia , lookup
Protein adsorption wikipedia , lookup
Proteolysis wikipedia , lookup
Two-hybrid screening wikipedia , lookup
Nuclear magnetic resonance spectroscopy of proteins wikipedia , lookup
Protein–protein interaction wikipedia , lookup
Protein mass spectrometry wikipedia , lookup
Protein purification wikipedia , lookup
Gel electrophoresis wikipedia , lookup
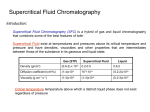
EUROPEAN COMMISSION JOINT RESEARCH CENTRE Institute for Reference Materials and Measurements IRMM Technical specifications for the supply, Installation and maintenance of a Capillary Electrophoresis System Including all related services and supplies IRMM / Ref. 2010-113-ic – technical specifications – capillary electrophoresis - p. 1 of 6 1. Introduction IRMM is one of the seven scientific research institutes of the Joint Research Centre (JRC) of the European Commission. The mission of IRMM is the development of a common and reliable European measurement system to support EC policy objectives. IRMM has four scientific departments and two supporting departments. IRMM employs some 330 , including 220 EC officials, the others being subdivided into visitors, post-doctoral fellows and temporary contract holders. Women represent approximately 30% of the staff. IRMM comprises analytical laboratories, facilities for the production of reference materials, radionucleide metrology and two accelerators for physical research. IRMM is an internationally recognised centre of excellence for measurement science. 2. Defining the market A contract notice was published in the Official Journal no. 2010/S 179-272275. 3. Subject of the market To carry out its mission, the IRMM requires the supply of an analytical platform for capillary electrophoresis techniques. The field of applications is the clinical chemistry and proteomics research, namely the identification and quantitation of proteins of interest (protein isoform profiling, and assessment of the purity of protein preparations). Capillary Electrophoresis (CE) is a well-established technique to separate and quantify proteins or other macromolecules (such as DNA, RNA) from complex mixtures. It also allowed the measurement of anions in solutions which can be interesting for example to determine the protein binding to metallic cations. The CE system is a computer assisted technique that regulates the current and/or the pressure which control the flow rate of a solvent (the electrolyte) through a specific column (the capillary). Separated and purified proteins are then analysed by UV detection at a specific wavelength or by their absorption spectrum (detection within a certain range of wavelengths by the mean of a diode array detector) or by fluorescence induced by irradiation with a laser light. Depending on the types of separation preferred, inner part of the capillary can be modified. CE can be performed both for identification purposes (molecular weight, ionisation potential) as well as for quantification. IRMM / Ref. 2010-113-ic – technical specifications – capillary electrophoresis - p. 2 of 6 4. Required characteristics Minimum requirements a) Yes No Capillary Electrophoresis (CE) system Capillary electrophoresis platform for protein and peptide identification and quantification where the configuration can be modified with automation kits and upgradable. The characteristics shall be the following: For our specific purposes, the system shall be capable of performing different techniques in particular for our applications : Capillary isoelectric focusing (cIEF) Sodium Dodecyl Sulfate Molecular Weight separation (SDS-MW) Integrated UV detector that can operate at typical wavelengths for proteins (200, 214, 254, 280 nm) Integrated Diode Array detector that can operate in the range from 190 to 600 nm. Integrated Laser Induced Detector with laser at 488 nm The system must be software monitored (see section d). Electrophoretic conditions : Voltage : up to 30 kV, current : up to 300 µA. Sample temperature control : 4-60 °C Capillary temperature control : 10-60 °C Voltage: 100-120 / 220-240 V Injection modes shall include pressure and electrokinetic Fluorescent IRMM / Ref. 2010-113-ic – technical specifications – capillary electrophoresis - p. 3 of 6 Page ref in tender (if applicable) Yes b) No CE-MS coupling The CE system is intended to be coupled to mass spectrometry detection: The compatibility with the two MS present in our laboratory (Applied Biosystem 4000 QTrap and ThermoScientific XT Vantage) shall be demonstrated. c) Consumables Consumables kit shall include at least: d) Sample trays Sample vials : 500 vials Buffer trays Buffer vials and caps Capillary cassettes A set of capillaries corresponding to our main applications (protein analysis with or without MS coupling). Capillary cutter Analysis software Analysis software capable of fast and efficient control of all the monitored parameters during protein measurement which shall be able to generate highquality analysis results. The software shall enable a real-time control of the CE system based on a dedicated controller interface with a computer based graphical user interface where methods and operation sequences could be fully programmed by the end user. IRMM / Ref. 2010-113-ic – technical specifications – capillary electrophoresis - p. 4 of 6 Page ref in tender (if applicable) Yes No Functions to be included: Real-time flow scheme. Display of all monitor values. Method Logbook for full documentation. Method start protocol. Note books for pre-run, run, and post-run notes. Calibration curves calculation with post-run quantification of unknown samples. Method handling with full flexibility. Backup of the different methods. e) Workstation for handling A PC controller that enables easy communication with the workstation and peripheral devices via an external USB communicator. The controller must include the Windows XP operating system or equivalent, the Capillary Electrophoresis application software, a QWERTY keyboard, a mouse, and a high-resolution flat-panel monitor. f) Warranty The candidate's offer shall include a warranty period of at least 24 months after delivery and acceptance of the requested equipments. g) Installation, operation qualification and training The supplier shall install the equipment at the premises of the IRMM and commission the instrument (operational qualification, including a report and validation of the software). IRMM / Ref. 2010-113-ic – technical specifications – capillary electrophoresis - p. 5 of 6 Page ref in tender (if applicable) Yes No Page ref in tender (if applicable) The supplier shall provide at least 1 day training at the premises of the IRMM. The price and description of the training has to be included in the offer. The training has to cover basic instrument maintenance as well as basic and advanced operation and software features. The training has to be given on site for at least 6 people. The training sessions have to be arranged by mutual agreement between the trainer and the trainees. The training has to be given in the English language. h) Maintenance service contract A preventive maintenance contract for 1 year, maximum 4 times extendable. The contract must cover preventive maintenance and operation qualification with at least one visit per year. The preventive maintenance service will start after the expiry of the warranty period. i) Application notes Documentation on the successful use of the CE system for protein characterisation and quantification. 5. Submitting an offer to the Commission The tenderer assures that he has proved the completeness of the content of this tender and that it is neither incomplete nor ambiguous. By signature, the tenderer admits that he acknowledges this tender as legally binding in case a Contract is awarded. ..........................................................,........................................ Place Date .................................................................................................................. The tenderer (Stamp and signature) IRMM / Ref. 2010-113-ic – technical specifications – capillary electrophoresis - p. 6 of 6