* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download RL 4 Chiral drugs
Toxicodynamics wikipedia , lookup
Discovery and development of tubulin inhibitors wikipedia , lookup
Discovery and development of angiotensin receptor blockers wikipedia , lookup
Discovery and development of proton pump inhibitors wikipedia , lookup
Orphan drug wikipedia , lookup
Pharmacokinetics wikipedia , lookup
Discovery and development of beta-blockers wikipedia , lookup
NK1 receptor antagonist wikipedia , lookup
Pharmacogenomics wikipedia , lookup
Prescription costs wikipedia , lookup
Pharmaceutical industry wikipedia , lookup
Drug design wikipedia , lookup
Prescription drug prices in the United States wikipedia , lookup
Drug discovery wikipedia , lookup
Psychopharmacology wikipedia , lookup
Neuropharmacology wikipedia , lookup
Pharmacognosy wikipedia , lookup
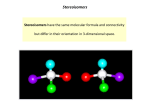
Reading to Learn Activity (4) Chiral Drugs What is chirality? chemical properties of diastereomers can differ and it is not unusual for them to have different melting and boiling points, refractive indices, solubility, etc. “Chirality” is the property possessed by a molecule with such spatial arrangement of atoms that it cannot superimpose on its mirror image. The object and mirrorimage pair of molecules has the same constituents and structural formula. Their relationship with each other is similar to our left and right hands. The carbon atom of a simple chiral centre has four different groups arranged tetrahedrally (Fig. 1). Isomers of such nature are called enantiomers. b b 3. c Fig.1: A chiral molecule with tetrahedral arrangement and its mirror image. There are three types of stereoisomers, namely enantiomers, diastereomers and geometrical isomers. 1. 2. 1' H CH3 2' H N H pseudoephedrine Fig. 2: The structure of diastereomeric molecules: ephedrine and pseudoephedrine. d c H ephedrine Chiral centre d OH CH3 2H N OH a a Chiral centre H 1 Geometrical isomers are molecules with a carbon-carbon double bond and they are not optically active. When the groups attached to each end of the double bond are on the same side, the stereoisomer is named as cis-isomer; when the groups are on the opposite sides, the trans-isomer designation is used. Why is chirality important in drug development? Enantiomers are two stereoisomers containing asymmetric carbon atoms related as non-superimposable object and mirror images. If an enantiomer rotates polarized light to the right or in a clockwise direction, it is said to be the (+) or the dextrorotatory isomer. On the other hand, if the plane polarized light is rotated to the left or in a counter-clockwise direction, the isomer is called as the (−) or the levorotatory isomer. Enantiomers are identical in chemical and physical properties except for the direction of rotation of plane polarized light. Biological systems like that of human beings have been known to exhibit chirality. This is reflected by the existence of chirality of drug receptor areas and the requirement of chiral specificity on drugs. In order to understand the biological effect of drugs, we have to distinguish the three main phases of their action. The first phase is the initial receptor differentiation phase, where different drugs have different affinity and tissue specificity due to receptor differentiation and distribution for the parent compound formed. The second phase is the absorption, distribution, metabolism and excretion phase, where the type of bioavailability is determined. The third phase is the interaction of the drug with the molecular site of action, leading to the observed Diastereomers are stereoisomers that are not related as object and mirror images. They contain at least two asymmetric carbon atoms. Unlike enantiomers, the physical and 147 Reading to Learn Activity (4) therapeutic effect. What drugs in our daily life are chiral? The three phases of action are based on the receptor theory, similar to the lock-and-key hypothesis proposed by the famous scientist Emil Fischer. Receptor molecules in the body are proteins that exhibit high affinities for the binding of molecules with certain structures. This is completely analogous to enzyme-substrate binding. Mismatching of drug molecules with the targeted receptors may cause undesirable side effects such as requirement of higher dosage and increased toxicity. Chirality is an essential dimension in pharmacology. Worldwide sales of chiral drugs in single-enantiomer forms continue to grow. The commonly used single-enantiomer drugs are grouped as follows: • • • All pharmacological activity may reside in one enantiomer. The therapeutic inactive isomer is regarded as an impurity that possesses a different or undesirable pharmacological entity. This situation may become even more acute if the active enantiomer exhibits a low therapeutic value or there is clinically significant toxicity. A well-known example of therapeutic-specific pair is the R- and S-enantiomers of thalidomide (the R and S designation, after Cahn, Ingold and Prelog, is a way of naming enantiomers by their structures). The R-enantiomer is an effective sedative, which is a medication with calming and soothing effect that relieves anxiety and promotes sleep. However, the S-enantiomer may cause teratogen formation. A teratogenic foetus is one with deficient, redundant, misplaced or grossly misshapen parts. S-Thalidomide was shown to be responsible for over 2,000 cases of serious birth defects in children born of women who took it during pregnancy. O • • Questions 1. 2. Name and draw structures for the geometric isomers of pent-2-ene. 3. 2,3-dihydroxybutanedioic acid (tartaric acid) is a chiral molecule having both enantiomers and diastereomers. Draw all possible stereoisomers for the compound and identify the chiral centres and the pair(s) of enantiomers. H O N NH O S-Thalidomide O N HN O Classify the following substances into chiral and achiral categories. basket ball gloves amino acids enzymes for digesting proteins fats pain killer drugs water O H O • Cardiovascular disease: Atorvastatin calcium, Simvastatin, Pravastatin sodium and Valsartan Central nervous system: Paroxetine hydrochloride and Sertraline hydrochloride Respiratory: Fluticasone propionate and Salmeterol Hematology: Clopidogrel bisulfate Gastrointestinal: Esomeprazole magnesium Antibiotic: Amoxicillin and potassium clavulanate O R-Thalidomide Fig. 3: The enantiomers of thalidomide 148 Reading to Learn Activity (4) 4. What are the advantages of providing drugs in enantiomerically pure form? How can single-enantiomeric drugs be obtained in practice? 5. Imagine that you can walk through a mirror into a mirror world where all objects, including atoms and molecules, exist as their mirror images. Assuming that you remain unchanged by this transition, i.e. enzymes in your body are not affected and are still capable of processing molecules in the same way as that in the parent world, what problem would you encounter during your stay in the mirror world? Explain briefly. Hint: In the mirror world, you would not obtain energy by taking sugar labeled as D-glucose because it is in fact L-glucose, the enzyme-inactive form of glucose. References 1. Roger Crossley. (1995). Chirality and the Biological Activity of Drugs. Boca Raton: CRC Press Inc. 2. Aboul-Enein & Wainer. (1997). The Impact of Stereochemistry on Drug Development and Use. Wiley International Publication. 3. Hava Caner, Efrat Groner & Liron Levy (2004). Trends in the Development of Chiral Drugs. Drug Discovery Today, Vol. 3 No. 3. 4. Gordon T. Yee (2002). Through the Looking Glass and What Alice Ate There. Journal of Chemical Education, No. 5, Vol. 79, 569 – 571. 5. Dinan F.J. &Yee G.T. (2004). An Adventure in Stereochemistry. Journal of College of Science Teaching, No. 2, Vol. 34, 25 – 29. 6. http://www.nlm.nih.gov/medlineplus/druginfo/medmaster/a600045.html 7. 芮耀誠 (2002)《實用藥物手冊》香港:中華書局。 8. http://www.atchem.net/Catalogalphabetic/cf_cz.HTM 149