* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download LECTURE11.SynapsesIV
Node of Ranvier wikipedia , lookup
Cyclic nucleotide–gated ion channel wikipedia , lookup
Signal transduction wikipedia , lookup
Mechanosensitive channels wikipedia , lookup
Endomembrane system wikipedia , lookup
Membrane potential wikipedia , lookup
Action potential wikipedia , lookup
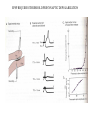
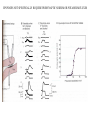
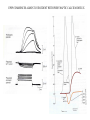
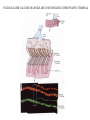
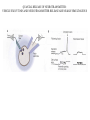
LECTURE 11: SYNAPSES IV: TRANSMITTER SYNTHESIS AND RELEASE REQUIRED READING: Kandel text, Chapters 14, 15 Giant synapse of squid used in classical experiments to determine the mechanism of chemical synaptic transmission Voltage Recording Current Injecting Pre Post Stimulating Electrode Voltage Recording EPSP REQUIRES THRESHOLD PRESYNAPTIC DEPOLARIZATION EPSP DOES NOT SPECIFICALLY REQUIRE PRESYNAPTIC SODIUM OR POTASSIUM FLUXES EPSP COMMENCES ALMOST COINCIDENT WITH PRESYNAPTIC CALCIUM INFLUX VOLTAGE-GATED CALCIUM CHANNELS ARE CONCENTRATED AT PRESYNAPTIC TERMINAL QUANTAL RELEASE OF NEUROTRANSMITTER AT NEUROMUSCULAR JUNCTION: STIMULUS-EVOKED mEPSPs ARE MULTIPLES OF SPONTANEOUS MINIATURE mEPSPs QUANTAL RELEASE OF NEUROTRANSMITTER: PRESYNAPTIC STIMULUS INDUCES RAPID SYNAPTIC VESICLE EXOCYTOSIS QUANTAL RELEASE OF NEUROTRANSMITTER: CALCIUM INFLUX AND VESICLE EXOCYTOSIS ARE NEARLY SIMULTANEOUS Membrane capacitance can be continuously sampled by sinusoidal voltage clamp Command Vm Ipipet Cm QUANTAL RELEASE OF NEUROTRANSMITTER: VESICLE EXOCYTOSIS AND NEUROTRANSMITTER RELEASE ARE NEARLY SIMULTANEOUS CALCIUM-MEDIATED SYNAPTIC VESICLE FUSION: REGULATION OF A BASAL VESICLE FUSION MACHINERY SYNAPSIN AND SYNAPTOTAGMIN ARE MEDIATORS OF CALCIUM REGULATION Synapsins restrain vesicles in a reserve pool. Synapsin phosphorylation by calcium/CAM-dependent protein kinase releases synapsin from vesicles. Synaptotagmin can bind to t-SNARE proteins SNAP25 and syntaxin, and also binds phospholipids in a calcium-dependent manner. MODULATION OF PRESYNAPTIC INTRACELLULAR CALCIUM DURING AXONAL ACTION POTENTIAL MODIFIES NEUROTRANSMITTER EXOCYTOSIS AND EPSP An action potential normally produces a transient increase in presynaptic calcium, which is dissipated by diffusion and calcium buffers. A high-frequency train of spike (tetanus) saturates the buffering capacity, creating a period of “potentiation”, where each action potential releases more neurotransmitter. Short-term potentiation, which does not require new protein synthesis lasts on the order of minutes. MODULATION OF PRESYNAPTIC INTRACELLULAR CALCIUM DURING AXONAL ACTION POTENTIAL MODIFIES NEUROTRANSMITTER EXOCYTOSIS AND EPSP Small changes in resting potential of presynaptic terminal dramatically affect resting [Ca+2]in by altering number of L-type Ca channels open at rest. These sub-threshold calcium levels combine with action potential-induced cacium influx in determining amount of synaptic vesicle exocytosis. L-type Ca channels regulated through axo-axonic serotonergic synapses. Alternatively, axo-axonic synapses can regulate K+ channels to determine duration of depolarization capable of activating calcium channels. SMALL-MOLECULE NEUROTRANSMITTERS Small molecule neurotransmitters are amino acids or metabolic products (usually of amino acids) generated by neuron-specific enzymes TRANSPORTERS PACKAGE SMALL-MOLECULE NEUROTRANSMITTERS INTO SMALL CLEAR VESICLES AND MEDIATE REUPTAKE FROM SYNAPTIC CLEFT Transporters are targets of many clinical drugs and drugs of abuse. Transporter-specific inhibitors (e.g., amphetamines) mediate prolonged postsynaptic stimulation by uncleared neurotransmitter. “False transmitter” is transmitter analog packaged into vesicles in place of endogenous transmitter, but with reduced or no ability to bind NT receptor. SMALL PEPTIDE NEUROTRANSMITTERS ARE GENERATED BY PEPTIDASES ACTING ON PACKAGED PROPEPTIDES (PROHORMONES) Propeptide is inserted into lumen of endoplasmic reticulum by N-terminal signal sequence. Peptidases act within Golgi to generate peptides, which are budded off to form large dense-core vesicles. Many propeptides can give each give rise to multiple peptide transmitters. Peptidases cleave N-terminal to adjacent pairs of basic residues (arginine, lysine) DIFFERENCES BETWEEN SMALL-MOLECULE AND PEPTIDE TRANSMITTERS PROPERTY Site of synthesis Mechanism of vesicle loading Vesicle type Vesicle localization NEUROTRANSMITTER TYPE SMALL-MOLECULE PEPTIDE Cytoplasm Pre-synaptic transporter Small clear (usually) Pre-synaptic Intravesicular Budding from Golgi Large dense-core Diffuse Exocytic mechanism Fast Slow Vesicle recycling Yes No Neurotransmitter recycling Yes No