* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download dual-center hypothesis
Survey
Document related concepts
Transcript
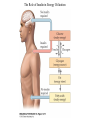
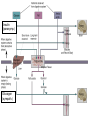
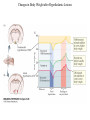
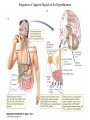
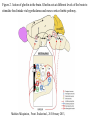
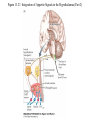
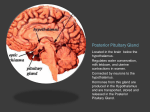
Nutrient Regulation Helps Prepare for Future Needs • Aside from obtaining energy from ingested food, nutrients are needed for growth, maintenance, and repair of the body. • • • • Such as 9 essential amino acids Some fatty acids Vitamins Minerals • The nervous system • Controls digestion • Monitors nutrient levels and energy balance • Anticipates future requirements Nutrient Regulation Helps Prepare for Future Needs Basal metabolism • Basal metabolism is energy used for heat production, maintenance of membrane potentials and life-sustaining processes. • Basal metabolism consumes around 55% of food energy – Around 12% for active behavioral processes • Which depends on activity level of the individual • Kleiber’s equation for the rate of basal metabolism—a rule that relates energy expenditure to body weight: – kcal/day = 70 × weight 0.75 – For individuals with normal caloric intake and activity levels • Restricting calories tends to lower basal metabolism • Restricting calories (50 -75 %) tends to increase longevity • Drugs to increase basal metabolism through changes in mitochondria? The Relation between Body Size and Metabolism Nutrient Regulation Helps Prepare for Future Needs Glucose is the principal sugar used for energy. Glycogen is a complex carbohydrate, made by the combining of glucose molecules, stored for a short term in the liver and muscles. Glycogenesis is the process of converting glucose to glycogen, regulated by the pancreatic hormone insulin, released by beta cells in the islets of Langerhans. Glucagon, another pancreatic hormone released by alpha cells in the islets of Langerhans, mediates glycogenolysis –conversion of glycogen back into glucose when blood glucose levels drop. Lipids (or fats) for longer-term storage, are deposited in adipose tissue. Gluconeogenesis is the process of converting fat and proteins to glucose and ketones, a form of fuel. The Role of Insulin in Energy Utilization Insulin Is Crucial for the Regulation of Body Metabolism • Glucose transporters span the cell membrane and interact with insulin to bring glucose into the cell. • Three sequential mechanisms trigger insulin release: • 1.The sensory stimulus of food evokes insulin release, in anticipation of glucose—the cephalic phase. • 2. Food causes gut hormone release, which stimulates the pancreas to secrete insulin—the digestive phase. • 3. Glucodetectors in the blood and liver detect glucose and signal the pancreas to release insulin—the absorptive phase. • Information from glucodetectors in the liver travels via the vagus nerve to the nucleus of the solitary tract (NST) in the hypothalamus. • This system informs the brain of glucose levels, and efferent fibers to the pancreas modulate insulin release. The Fasting and Absorptive Phases of Metabolism Insulin (parasymp.) Glucagon (sympath.) Regulation of Eating • Satiety is the feeling of fulfillment or satisfaction. • Hunger is the internal state of an animal seeking food. • The brain integrates insulin and blood glucose levels with other information to decide whether to initiate eating. • No single brain region has control of appetite, but the hypothalamus is important to regulation of: – Metabolic rate – Food intake – Body weight • A dual-center hypothesis proposed two appetite centers in the hypothalamus: (now considered outdated) – One for signaling hunger – One for signaling satiety The Hypothalamus Coordinates Multiple Systems That Control Hunger • Ventromedial hypothalamus (VMH) lesions cause animals to eat to excess (hyperphagia) and become obese, suggesting the VMH is a satiety center. • Lateral hypothalamus (LH) lesions cause aphagia—refusal to eat—suggesting LH is a hunger center. • However, the dual-center hypothesis proved to be too simple. • VMH-lesioned animals exhibit a dynamic phase of obesity with hyperphagia (overeating) until they become obese, on a rich diet. • Their increased weight stabilizes in a static phase of obesity; this weight is maintained even after food manipulations. Changes in Body Weight after Hypothalamic Lesions Integration of Appetite Signals in the Hypothalamus The Hypothalamus Coordinates Multiple Systems That Control Hunger • The arcuate nucleus of the hypothalamus contains an appetite control circuits governed by hormones, such as insulin. • Other peripheral peptide hormones are • Leptin, • Ghrelin • PYY3–36. Role of Leptin • Fat cells produce leptin and secrete it into the bloodstream. • Leptin works to suppress hunger – Leptin receptors (ObR) are located in the hypothalamus • Leptin’s effects on the arcuate neucleus are long-lasting. • Leptin activates POMC/CART neurons but inhibits NPY/AgRP neurons CNS leptin and insulin action in the control of energy Homeostasis (2010) Bengt F. Belgardt and Jens C. Bruning, Annals of the New York Academy of Sciences Volume 1212, Issue 1, CNS Leptin and Insulin Role of Ghrelin • • • • • Peptide hormone released from cells in the stomach Increases growth hormone secretion (GH-releasing) Increases during fasting and decreases after a meal Increased levels produce increased appetite Receptors located in the arcuate nucleus, lateral hypothalamic area, accumbens nucleus, ventral tegmental area • Obese individuals – have low baseline levels – levels do not drop after a meal so no signal for “just ate a meal” Figure 2. Action of ghrelin in the brain. Ghrelin acts at different levels of the brain to stimulate food intake via hypothalamus and meso-cortico-limbic pathway. Mathieu Méquinion, Front. Endocrinol., 26 February 2013, Role of PYY3-36 • Pancreatic Peptide Tyrosine Tyrosine • Small peptide from the small intestine – from cells in the ileum and colon – low baseline levels that increase quickly when eating • Increased levels decrease appetite • Receptors located in the arcuate nucleus and vagus nerve input to the brainstem Figure 13.23 Integration of Appetite Signals in the Hypothalamus (Part 2) - + - The Hypothalamus Coordinates Multiple Systems That Control Hunger • Hypothalamic mechanisms integrate appetite signals. • The paraventricular nucleus (PVN) and the lateral hypothalamus (LH) are primary targets of the arcuate nucleus. • Orexigenic neurons of the lateral hypothalamus act to increase appetite and food intake, whereas anorexigenic neurons of the PVN act to decrease appetite and feeding. • Orexin “hypocretin” is a peptide produced in the LH • that regulates arousal, wakefulness, and appetite The Hypothalamus Coordinates Multiple Systems That Control Hunger The Nucleus of the Solitary Tract (NST) in the brainstem receives and integrates appetite signals from many sources, some via the vagus nerve. Cholecystokinin (CCK) is a peptide hormone released by the gut after high intake and acts on receptors on the vagus nerve to inhibit appetite. Regulation of Energy Intake Is A Complex Process • Integration of multiple signals from • Internal homeostatic mechanisms • External sensory cues • • • • • social context availability of food learned behaviors cognitive factors habits Cognitive and Emotional Influences on Eating • Cognitive – Sensory • Taste & Odor • Visual – Memory • Early childhood eating habits • Food preferences generally • Cultural influences • Emotional – Food sensory input can activate • Reward system • Disgust system – Negative emotions • Fear, sadness, anger can disrupt eating • Sometimes increases and sometimes decreases eating Role of Learning in Eating • Learning can influence eating in a variety of ways • Most mammals are born with a preference for sweet and salty tastes and with an aversion to bitter tastes • Learn to avoid any taste followed by illness – conditioned taste aversion • Learn to prefer tastes that improve their health – conditioned taste preference • These forms of learning are robust and adaptive Positive-Incentive Models of Feeding • Many brain circuits are activated in response to seeing food cues, – – – – – – – – Prefrontal cortex Orbitofrontal cortex Inferior temporal cortex Insula Striatum Amygdala Hippocampus Hypothalamus • High hedonic value food produces greater activation of the brain circuits Positive-Incentive Models of Feeding • Major influences of taste, learning & social factors on feeding • Alternative theory of feeding & hunger – based on idea that we eat because eating is pleasurable rather than to satisfy some setpoint for glucose or fat. – When good food is present, we will eat regardless – Hunger determined by many factors • • • • • Taste Previous experience with food Time of day Time since last meal Social environment Sensory Signals and Positive Incentives • The homeostasis “set point” explanation of eating regulation can not explain eating a piece of pecan pie and whipped cream at the end of a large meal • In rats, a small amount of artificial sweetener saccharin added to their diet leads to an increase in consumption and marked weight gain • Positive-incentive properties of food (i.e., anticipated pleasurable effects) rather than internal deficits • Deprivation increases food's positive incentive properties • Positive energy balance “Over eating” reduce food’s incentive signals especially in the Insula and Hypothalamus – For individuals with good regulation i.e. “thin” – But not for individual’s whom tend to be overweight Sensory-Specific Satiety • Eating one particular food (chocolate cookie) reduces incentive value of its taste • Cafeteria diet has variety so incentive value does not drop as quickly – rats increase consumption & body weight – many choices allows switching as incentive value for a particular food falls