* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Sample Abstract
Survey
Document related concepts
Transcript
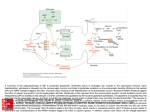
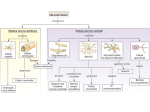
(Recent Photograph) Sample Abstract (List of items to include in your abstract) 1. Abstract Title: Mechanisms of cerebral hyperexcitability induction by antiviral acute phase response 2. All Authors: Miranda Reed, PhD, Auburn University; Holly Hunsberger, PhD, Auburn University, Gregory Konat, PhD, West Virginia University Presenters: Miranda Reed, PhD, Auburn University 3. 4. Short Description of what will be discussed during the presentation A body of clinical evidence has demonstrated that viral infections in the periphery exacerbate neurodegenerative conditions, e.g., multiple sclerosis, Parkinson disease, Alzheimer Disease (AD), and seizures. For example, peripheral infections increase the propensity and severity of seizures in susceptible populations, and AD patients suffering from systemic infections develop symptoms earlier and exhibit greater cognitive impairments than patient with less infectious insults. Although experimental studies strongly indicate the initial response of the innate immune system to infection, i.e., the acute phase response (APR), as the primarily trigger, the underlying mechanisms have not been defined. In a quest to elucidate these mechanisms, we have shown that APR instigated by a viral mimic, polyinosinicpolycytidylic acid (PIC), elicits protracted hyperexcitability of cerebral networks by robustly increasing the levels of resting extracellular glutamate. These levels gradually return to baseline values within four days. While pre-synaptic potassium-evoked glutamate release is not affected, glutamate uptake is profoundly impaired and non-vesicular glutamate release is augmented, indicating functional alterations of astrocytes. Electrophysiological examination of hippocampal slices from PIC-challenged mice reveals a several fold increase in the basal synaptic transmission as compared to control slices. PIC challenge also increased the probability of pre-synaptic glutamate release as seen from a reduction of paired-pulse facilitation. Furthermore, twenty-four hours after PIC challenge, the mice feature an approximately 3-fold increase in cumulative seizure scores and 2-fold increase in the duration of status epilepticus induced by subcutaneous (s.c.) injection of 12 mg/kg of kainic acid. Seizure scores positively correlated with pre-seizure tonic glutamate. Moreover, seizures resulted in a profound (76%) elevation of extracellular glutamate in the CA1 of PIC-challenged but not saline-injected mice. Our results implicate the increase of extracellular glutamate as a mediator of seizure hypersusceptibility induced by peripheral viral challenge. Finally, our findings that similar alterations in glutamatergic functioning (i.e., increased extracellular levels and decreased glutamate uptake) are observed in mouse models of AD early suggests one possible mechanism by which peripheral infections accelerate the onset and progression of AD. 5. What will the audience take away from your presentation? (Try to list 3-5 specific items) Explain how the audience will be able to use what they learn? The elucidation of underlying mechanisms by which peripheral infections increase hyperexcitability will vertically advance and expand understanding of the mechanistic link between viral infections in the periphery and the exacerbation of neurodegeneration, an important consideration given the number of neurodegenerative diseases in which increased glutamatergic signaling is observed. The proposed study is significant because it will provide a foundation for the development of innovative therapeutic strategies to attenuate or even prevent the progression of neurological deficits in patients afflicted with neurodegenerative disorders. How will this help the audience in their job? Is this research that other faculty could use to expand their research or teaching? Does this provide a practical solution to a problem that could simplify or make a designer’s job more efficient? Will it improve the accuracy of a design, or provide new information to assist in a design problem? List all other benefits. In addition to the general knowledge, we will discuss a novel method to examine glutamatergic levels in awake, behaving animals. This methodology allows for correlation with behavioral tasks, allowing for more nuanced experimental questions. 6. Is this abstract connected to an organized session? If yes, please provide full session title. No 7. Biography of presenting author (about 100 words) Dr. Miranda N. Reed is an Associate Professor of Drug Discovery and Development at Auburn University. Trained in psychology, neuroscience, and neurology, her postdoctoral studies focused on the consequences of tau mislocalization into dendritic spines in the context of Alzheimer’s disease at University of Minnesota under the direction of Karen Hsiao Ashe. Her current work focuses on alterations in glutamatergic functioning and the resultant consequences of these alterations in various neurodegenerative conditions, including Alzheimer’s disease and seizures. Author Details: Full Name: Miranda Nicole Reed Contact Number: 334-844-7401 Country: USA Category: Oral Session Name: Neurodegenerative conditions Email: [email protected]