* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download atom
Survey
Document related concepts
Transcript
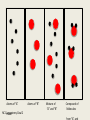
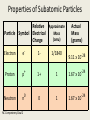
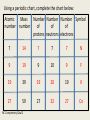
Atomic Structure NC Competency Goal 2 A History of the Atom Atomic Structure • By the late 1800’s scientist were convinced that atoms were the fundamental units of which all matter is composed. • They soon learned that the atom was not indivisible. • Atoms are composed are smaller (subatomic) particles: proton, neutron, and electron NC Competency Goal 2 Atoms An atom is the smallest particle of an element that retains the properties of that element. Atoms are extremely small. One gram of Pb (lead) has 2.9 x 1021 atoms. Compare that to the Earth’s population which is about 4 x 109 people. So…….where did the idea of atoms come from? NC Competency Goal 2 Atoms The idea of atoms were first suggested by an ancient Greek citizen named Democritus. The word “atom” comes from the Greek language: Atomos = Indivisible NC Competency Goal 2 Atoms It wasn’t until the late 1700’s that chemist were able to relate chemical changes to events at the level of individual atoms. At that time, an English chemist and physicist named John Dalton (1766-1844) first stated his atomic theory. NC Competency Goal 2 Atoms: Daltons Atomic Theory 1-All elements are composed of tiny indivisible particles called atoms. 2-Atoms of the same element are identical. The atoms of any one element are different from atoms of another element. 3- Atoms of different elements can combine in simple whole number ratios to form compounds. 4 – Chemical reactions occur when atoms are separated, joined or rearranged. However, atoms of one element are not changed into atoms of another element by a chemical reaction. NC Competency Goal 2 Atoms of “A” NC Competency Goal 2 made Atoms of “B” Mixture of “A” and “B” Compound of Molecules Electrons, Protons, and Neutrons *Electrons are negatively charged subatomic particles. *They were primarily the interest of electricians rather than chemist. *These scientists (i.e. electricians) studied the flow of gases in an enclosed tube…at low pressure. The tube had two metal plates at each end. One of the plates held a positive charge and the other a negative charge. When high voltage was applied, the gas inside the tube glowed. NC Competency Goal 2 NC Competency Goal 2 The device they used is called a cathode ray tube. The glowing beam inside the tube is called a cathode ray. Electrons (which are negatively charged are attracted to the positive plate) hence electrons travel from the negative cathode toward the positive anode. NC Competency Goal 2 An English physicist named Sir Joseph J. Thomson (18561940) experimented with Cathode rays. In 1897, he found that cathode rays could be deflected by a magnet or an electrically charged plate place within or near the Cathode ray tube. NC Competency Goal 2 Cathode rays are a collection of very small negatively charged particles. He called these particles Electrons. He found out the following: J.J. Thomson • The mass of an electron is lighter than a hydrogen atom. • No matter what gas was used in the cathode ray tube or the type of metal used for electrodes within the tube…cathode rays are ALWAYS made of electrons. NC Competency Goal 2 J.J. Thomson believed electrons exisited in ALL atoms! J.J. Thomson His model of the atom became known as the “plum pudding” model of the atom! NC Competency Goal 2 Hydrogen Atom Q: If atoms are electrically neutral, what would happen to a hydrogen atom if it lost it’s only electron? Answer: It would exist as a bare proton. NC Competency Goal 2 Hydrogen Atom A single proton is 1840 x heavier than an electron. A proton carries a single positive charge. NC Competency Goal 2 In 1932 the physicist, James Chadwick (1891 – 1974) confirmed the existence of yet another subatomic particle: the neutron. * Neutrons are subatomic particles with NO charge. * They reside with protons in the nucleus. The mass of a neutron is nearly equal to the mass of a proton! NC Competency Goal 2 At this point, the fundamental building blocks of an atom were in existence! The fundamental building blocks of an atom are: Protons, neutrons, and electrons. NC Competency Goal 2 In 1886, Eugen Goldstein observed rays traveling in the opposite direction of the cathode ray. This occurred when used a cathode with holes in it. NC Competency Goal 2 He later called these rays, “Canal Rays”. Later, the canal rays were found to be made up of positively charged particles. NC Competency Goal 2 Eugen Goldstein If the cathode ray tube contained Hydrogen gas, then the canal rays were made up of bare protons! Eugen Goldstein NC Competency Goal 2 Properties of Subatomic Particles Relative Approximate Mass Particle Symbol Electrical (amu) Charge Actual Mass (grams) Electron e- 1- 1/1840 9.11 x 10-28 Proton p+ 1+ 1 1.67 x 10-24 Neutron n0 0 1 1.67 x 10-24 NC Competency Goal 2 The discovery of the nucleus (which houses protons and neutrons) is credited to Ernest Rutherford. Click the link below to view Rutherford’s now famous “Gold Foil Experiment”. Click Here to see Rutherford’s Gold Foil Experiment NC Competency Goal 2 The Structure of the Atom The nucleus of an atom is protons composed of ____________ neutrons and _____________. positive The nucleus has a ___________ charge and occupies a very small part of the volume of an atom. NC Competency Goal 2 Atomic Number The atomic number of an element is the number of protons in the nucleus of an atom for that element. Since atoms are electrically neutral, the number of protons in the nucleus of an atom must equal the number of electrons orbiting the nucleus NC Competency Goal 2 Atomic Number Q: Element Carbon is atomic number 6. How many protons and electrons are in a carbon atom? + and 6 e6 p Answer: ______________________________ NC Competency Goal 2 Atomic Number Q: The atomic number of an element is 11. What is the element? Sodium Answer: ______________________________ NC Competency Goal 2 REVIEW Who am I ? I’m the first to develop the concept of atoms. I am a Greek philosopher. Democritus Answer: ______________________________ NC Competency Goal 2 REVIEW Who am I ? I developed an early atomic theory that stated atoms can combine with each other to form new compounds. John Dalton Answer: ______________________________ NC Competency Goal 2 REVIEW Who am I ? I “coined” the term “electrons” and designed the “plum pudding” model of the atom. J.J. Thomson Answer: ______________________________ NC Competency Goal 2 REVIEW Who am I ? I am an English physicist who discovered the neutron. James Chadwick Answer: ______________________________ NC Competency Goal 2 REVIEW Who am I ? I designed a simple experiment known as the gold foil experiment. I am credited with the discovery of the nucleus. Ernest Rutherford Answer: ______________________________ NC Competency Goal 2 REVIEW Who am I ? I developed what is known as the “planetary model” of the atom. Neils Bohr Answer: ______________________________ NC Competency Goal 2 REVIEW What is the term used to describe the number of protons in the nucleus of an atom? Atomic Number Answer: ______________________________ NC Competency Goal 2 REVIEW Which subatomic particle is negatively charged and orbits the nucleus of an atom? The electron Answer: ______________________________ NC Competency Goal 2 REVIEW Which subatomic particle has no charge and is usually found in the nucleus of an atom? The neutron Answer: ______________________________ NC Competency Goal 2 REVIEW Which subatomic particle has a positive charge and is found in the nucleus of an atom? The proton Answer: ______________________________ NC Competency Goal 2 Mass Number • Most of the mass of an atom is concentrated in the nucleus. • The mass number is the total number of protons and neutrons in the nucleus. NC Competency Goal 2 Using a periodic chart, complete the chart below: Atomic Mass Number Number Number Symbol number number of of of protons neutrons electrons 7 14 7 7 7 N 9 19 9 10 9 F 19 39 19 20 19 K 27 59 27 32 27 Co NC Competency Goal 2 End of Review • Great Job! Now let’s continue our lesson… NC Competency Goal 2 Isotopes • Isotopes are atoms! • They belong to the same element! • Because they belong to the same element they have the same number of protons…..BUT they have a different number of neutrons Greek: Isotopos = the same place Atoms that have the same number of protons but a different number of neutrons are called Isotopes. NC Competency Goal 2 Isotopes Different Number ofProtons Neutrons Atoms Same of Number the same of element 6 p+ 6 n0 6 p+ 7 n0 6 p+ 8 n0 6 e- 6 e- 6 e- Carbon-12 Carbon-13 Carbon-14 98.89% NC Competency Goal 2 1.10% .01% Symbols for Isotopes p+ Mass Number 6 e- Atomic Number 6 6 n0 Carbon-12 NC Competency Goal 2 12 6 Check your Understanding Write the symbol for the following isotopes. Oxygen – 16 Oxygen – 17 Oxygen – 18 16 17 18 8 8 8 NC Competency Goal 2 Check your Understanding Determine the number of neutrons in Nitrogen-18. 18 7 NC Competency Goal 2 11 Neutrons Atomic Mass The atomic mass of an element is an “average” of many numbers. That’s why it doesn’t appear as a “whole number” on the periodic chart. Atomic Mass is defined as the weighted average of the masses of the isotopes of that element. NC Competency Goal 2 Atomic Mass Example Problem: Element X has two natural isotopes. The isotope with a mass number of 10 has a natural abundance of 20%. The isotope with a mass number of 11 has a relative abundance of 80%. Estimate the average atomic mass for the element from these figures. What is the true identity of element X? NC Competency Goal 2 Atomic Mass Example Problem Solution: 10X 11X 10 amu x .20 = 2.0 amu 11 amu x .80 = 8.8 amu Total 10.8 amu The average atomic mass of element X is 10.8 amu. Element X is Boron. Boron also has an atomic number of 5 NC Competency Goal 2 Atomic Mass Hydrogen has three naturally occurring isotopes. 1 p+ 0 n0 1 eHydrogen-1 Since most of the hydrogen atoms found in + + 1 p 1 p nature are Hydrogen-1 0 1n 2 n0 isotopes, the average atomic- mass of Hydrogen 1 e1e calculates to be Hydrogen-2 Hydrogen-3 1.0079 amu.Tritium Deuterium