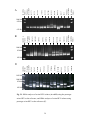
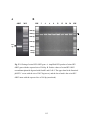
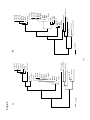
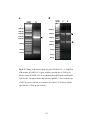
* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Cross-Species Infection and Characterization of Avian Hepatitis E
Leptospirosis wikipedia , lookup
Oesophagostomum wikipedia , lookup
2015–16 Zika virus epidemic wikipedia , lookup
Trichinosis wikipedia , lookup
Ebola virus disease wikipedia , lookup
Human cytomegalovirus wikipedia , lookup
West Nile fever wikipedia , lookup
Middle East respiratory syndrome wikipedia , lookup
Orthohantavirus wikipedia , lookup
Marburg virus disease wikipedia , lookup
Antiviral drug wikipedia , lookup
Herpes simplex virus wikipedia , lookup
Swine influenza wikipedia , lookup
Influenza A virus wikipedia , lookup
Lymphocytic choriomeningitis wikipedia , lookup
Hepatitis C wikipedia , lookup
Cross-Species Infection and Characterization of Avian Hepatitis E Virus By Zhifeng Sun Dissertation submitted to the Faculty of the Virginia Polytechnic Institute and State University in partial fulfillment of the requirements for the degree of Doctor of Philosophy In Veterinary Medical Sciences Department of Biomedical Sciences and Pathobiology Virginia-Maryland Regional College of Veterinary Medicine Dr. Xiang-Jin Meng, Chair Dr. S. Ansar Ahmed Dr. William R. Huckle Dr. F. William Pierson Dr. Thomas E. Toth Dr. Zhijian Tu January 20, 2005 Blacksburg, Virginia Keywords: hepatitis E, hepatitis E virus, avian HEV, swine HEV, heteroduplex mobility assay, cross-species infection, open reading frames 2 and 3, virion, turkey Copyright 2005, Zhifeng Sun Cross-Species Infection and Characterization of Avian Hepatitis E Virus Zhifeng Sun Abstract As novel or variant strains of HEV continue to evolve rapidly both in humans and other animals, it is important to develop a rapid pre-sequencing screening method to select field isolates for further molecular characterization. Two heteroduplex mobility assays (HMA) were developed to genetically differentiate field strains of swine HEV and avian HEV from known reference strains. It was shown that the HMA profiles generally correlate well with nucleotide sequence identities and with phylogenetic clustering between field strains and the reference swine HEV or avian HEV strains. Therefore, by using different HEV isolates as references, the HMA developed in this study can be used as a pre-sequencing screening tool to identify variant HEV isolates for further molecular epidemiological studies. Our previous study showed that avian HEV antibody is prevalent in apparently healthy chickens. A prospective study was conducted on a known seropositive but healthy chicken farm. Avian HEV was identified from the healthy chicken flock. Avian HEV isolates recovered from the healthy chicken share 70-97% nucleotide sequence identities with those isolates which cause hepatitis-splenomegaly (HS) syndrome based on partial helicase and capsid gene regions. Recovery of identical viruses from the experimentally inoculated chickens in the subsequent transmission study further confirmed our field results. The capsid gene of avian HEV isolates from chickens with HS syndrome were also characterized and found to be heterogeneic, with 76-100% nucleotide sequence identities to each other. The study indicates that avian HEV is enzootic in chicken flocks and spread subclinically among chicken populations, and that the virus is heterogeneic. As HEV can not be propagated in vitro, in order to further characterize avian HEV, an infectious viral stock with a known infectious titer must be generated. Bile and feces collected from specific-pathogen-free (SPF) chickens experimentally infected with avian HEV were used to prepare an avian HEV infectious stock. The infectivity titer of this infectious stock was determined, by intravenously inoculating one-week old SPF chickens, to be 5 x 104.5 50% chicken infectious doses (CID50) per ml. Seroconversion, viremia as well as fecal virus shedding were observed in the inoculated chickens. Contact control chickens also became infected via direct contact with inoculated ones. Avian HEV infection in chickens was found to be dose-dependent. To determine if avian HEV can infect across species, one-week old SPF turkeys were intravenously inoculated each with 104.5 CID50 of avian HEV. The inoculated turkeys seroconverted to avian HEV antibodies at 4-8 weeks postinoculation (WPI). Viremia was detected at 2-6 WPI, and fecal virus shedding at 4-7 WPI in inoculated turkeys. This is the first demonstration of cross-species infection by avian HEV. Little is known regarding the characteristics of the small ORF3 protein largely due to the lack of a cell culture system for HEV. To characterize the small protein, the ORF3 proteins of avian HEV and swine HEV were expressed in Escherchia coli, and purified by BugBuster His-Bind Purification System. Western blot analysis showed that avian HEV ORF3 protein is unique and does not share common antigenic epitopes with those of swine HEV and human HEV. However, swine HEV (genotype 3) and human HEV (genotype 1) ORF3 proteins cross-react with each other antigenically. To determine if the ORF3 protein is a virion protein, infectious stocks of avian HEV and swine HEV were first generated in SPF chickens and pigs, respectively. Virions were subsequently purified by sucrose density gradient centrifugation and virion proteins were characterized by SDS-PAGE and Western blot analysis. Two major forms of ORF2 proteins of avian HEV were identified: a 56 kDa and an 80 kDa proteins. Multiple immunoreactive forms of ORF2 proteins of swine HEV were also observed: 40 kDa, 53 kDa, 56 kDa and 72 kDa. However, the ORF3 protein was not detected from the native virions of avian HEV or swine HEV. These findings provide direct evidence that ORF2 indeed encodes a structural protein of HEV, whereas ORF3 does not. iii To search for other potential animal reservoirs for HEV, the prevalence of IgG anti-HEV antibody was determined in field mice caught in chicken farms to assess the possibility of mice as a potential reservoir for HEV infection in chickens. Three different recombinant HEV antigens derived from avian HEV, swine HEV, and human HEV were used in the ELISA assays. The anti-HEV seropositive rates in wild field mice (Mus musculus), depending upon the antigen used, are 15/76 (20%), 39/74 (53%), and 43/74 (58%), respectively. HEV RNA was also detected from 29 fecal and/or serum samples of mice. The HEV sequences recovered from field mice shared 72-100% nucleotide sequence identities with each other, 73-99% sequence identities with avian HEV isolates, and 51-60% sequence identities with representative strains of swine and human HEVs. However, attempts to experimentally infect laboratory mice (Mus musculus) with the PCR-positive fecal materials recovered from the wild field mice were unsuccessful. We also attempted to experimentally infect 10 Wistar rats each with avian HEV, swine HEV, and an US-2 strain of human HEV, respectively. However, the inoculated rats did not become infected as evidenced by the lack of viremia, virus shedding in feces or seroconversion. These data suggest that mice caught in chicken farms are infected by a HEV-like virus, but additional work is needed to determine the origin of the mouse virus as well as the potential role of rodents in HEV transmission. In summary, we developed two HMAs which are useful for differentiation and identification of variant strains of swine and avian HEVs. We genetically identified and characterized an avian HEV strain from apparently healthy chickens in seropositive flocks. We showed that avian HEV can cross species barriers and infect turkeys. Our data indicated that avian and swine HEV ORF2 genes encode structural proteins, whereas ORF3 genes do not. Evidence in this study also showed that HEV or HEV-like agent exists in field mice on a chicken farm. iv DEDICATION This dissertation is dedicated to my parents, Yintang Sun and Songzhi Liu, and my wife, Mei Yu, as well as my sons, Brian and Edward. With love and appreciation. v ACKNOWLEDGEMENTS Thanks must begin with the person to whom I am most deeply indebted - my advisor, my mentor, Dr. Xiang-Jin Meng. His dependable presence as both a leader and friend provided me the encouragement and fortitude to complete this challenge. I cannot imagine the path I would have followed without his undying guidance and patience. I would also like to recognize my committee members Dr. Xiang-Jin Meng, Dr. Thomas E. Toth, Dr. S. Ansar Ahmed, Dr. F. William Pierson, Dr. William R. Huckle and Dr. Zhijian Tu for their advice, encouragement and service. A special expression of gratitude goes to Dr. Calvert T. Larsen for his friendship and contribution to my projects. I would also like to sincerely thank the gracious assistance provided by the following individuals for their technical support: Dr. Nammalwar Sriranganathan and Dr. David M. Moore (assistance in rodent study), Dr. Wen Li, Dr. Stephen M. Boyle, Dr. Mohamed Seleem, Dr. Abey Bandara and Dr. Jane A. Duncan (technical assistance), Julie Tucker (ultracentrifugation), Mary Nickle, Chris Wakley and Steve J. Salmon (animal care and sample collection), Gerald L. Baber (reprographic support) and Terry Lawrence (poster printing), and the Core Laboratory Facility at Virginia Bioinformatics Institute for assistance with DNA sequencing. So many thanks also go to my lab co-workers in Molecular Virology Laboratory and my colleagues and friends at CMMID, Denis Guenette, Kijona Key, Yaowei Huang, FangFang Huang, Padma Billam, Martjin Fenaux, Sheela Ramamoorthy, Nicole McKeown, Trevor Williams and Kerri Cooper, who have endured this endeavor with me. They have all been with me and helped me in ways too numerous to mention. I would also like to thank Dr. John C. Lee and Dr. Ludeman A. Eng for their support. I also thank the staff in the Office of Research and Graduate Studies and the Department of Biomedical Sciences and Pathobiology for their assistance. I would like to thank the administrators and faculty members that I have had the pleasure of learning from and working with over the past 4 1/2 years while at Virginia Tech. I would like to express my appreciation to Dr. Suzanne Emerson from the National Institutes of Health, Bethesda, Maryland for providing the mono-specific rabbit anti-HEV vi ORF3 peptide serum and rhesus macaque convalescent serum against ORF3 protein of human HEV, and Dr. En-Min (Eric) Zhou from Iowa State University, Ames, Iowa for providing the mono-specific rabbit antiserum against ORF2 peptide of avian HEV. I thank Dr. H. L. Shivaprasad and Dr. P. R. Woolcock of the California Animal Health and Food Safety Laboratory System, Fresno, CA for providing the chicken clinical samples. I am also grateful to Dr. Dean Erdman, Dr. Paul Rota and Dr. Thomas Ksiazek of the Centers for Disease Control and Prevention, Atlanta, GA for generously providing SARS-CoV RNA and SARS convalescent-phase patient serum. I would especially like to thank my family: My parents, my brother and sisters for their continued moral encouragement and support, and my wife, Mei Yu, who has always been there for me with her love and continued support, and my sons, Brian and Edward for being healthy and cute. This research was supported by grants from the National Institutes of Health (AI 01653, AI 46505 and AI 50611) and from the U.S. Department of Agriculture (NRI 3520412531). vii TABLE OF CONTENTS Abstract…………………………………………………….………………......ii Dedication………………………………………...………...………………......v Acknowledgements………………………………………….….….……..…...vi Table of Contents……………………………………………...………….....viii List of Figures………………………………………………….….……...….xiv List of Tables……………………………………………………..…….….…xvi General Introduction…………………………………………………....…xviii Chapter 1. Literature Review………………………………………..………..1 1.1 Introduction………………………………………………………….…...1 1.2History…………………………………………………………………….1 1.3 Virology…………………………………………………………...……...2 1.4 Molecular Characteristics………………………………………...……...3 1.5 Animal Models…………………………………….…………..………….4 1.6 Epidemiology and Seroprevalence……………………….………..……..4 1.6.1 Epidemiological Features and Transmission Routes………………...……...4 1.6.2 Cross-Species Infections…………………..………………...…….…..…5 1.6.3 Animal Reservoirs………………….………………………………...…6 1.7 Clinical Features………………………………………….…………..….6 1.8 Pathogenesis………………………………….………………………..…7 1.9 Diagnosis……………………………………….………………………...8 1.10 Prevention and Vaccines…………………….……………………..…...8 1.11 Swine Hepatitis E Virus (Swine HEV) ……………………………….…9 1.12 Avian Hepatitis E Virus (Avian HEV) ……………………………...…10 1.13 References…………………………..…………………………….……11 viii Chapter 2. Use of Heteroduplex Mobility Assays (HMA) for PreSequencing Screening and Identification of Variant Strains of Swine and Avian Hepatitis E Viruses (Published in Veterinary Microbiology. Sun et al. 2003)………………………………………………………….....…..23 2.1 Abstract……………………………………………………………..…..23 2.2 Introduction……………………………………………………….....…25 2.3 Materials and Methods….…………………………………………..….26 2.3.1 Virus Isolates…………………………………………………….…..26 2.3.2 RT-PCR Amplification of Different Swine and Avian HEV Isolates………...27 2.3.3 HMA of Swine and Avian HEV Isolates…………………………….…...27 2.3.4 Correlation of HMA Profiles with Sequence Analyses of Amplified Genomic Region(s) of Swine and Avian HEV Isolates………………....….28 2.3.5 Correlation of HMA Results with Phylogenetic Analyses of the Sequences of Swine and Avian HEV Isolates…………………………….28 2.3.6 GenBank Accession Numbers…………………………………..………29 2.4 Results……………………………………………………………….…29 2.4.1 HMA of Swine and Avian HEV Isolates with Respective Reference Viruses…………………………………………………………..…..29 2.4.2 Correlation of HMA Results with Percentage of Sequence Identities of Amplified Genomic Region(s) of Swine and Avian HEV Isolates………...30 2.4.3 Correlation of HMA Results with Phylogenetic Analyses of Swine and Avian HEV Sequences of Targeted Genomic Region(s)………….…....31 2.5 Discussion…………………………………………….…………..……31 2.6 Acknowledgements…………………………………….…….…………33 2.7 References……………………………………………….……….…….34 2.8 Figures………………………………………………….……………...39 2.9 Tables…………………………………………………….…………….42 Chapter 3. Genetic Identification of An Avian Hepatitis E Virus (HEV) from Healthy Chicken Flocks and Characterization of the Capsid Gene of 14 Avian HEV Isolates from Chickens with Hepatitis-Splenomegaly Syndrome in Different Geographic Regions of the United States (Published in Journal of General Virology. Sun et al. 2004)...……………..…43 3.1 Abstract…………………………………..……………………….……..43 3.2 Introduction………………………………………………….……..…...45 3.3 Materials and Methods…………………………………………...……..46 3.3.1 Clinical Samples………………………………………………...…….46 3.3.2 Prospective Study…………………………………………………...…47 ix 3.3.3 Primer Design……………………………………………………...…47 3.3.4 RT-PCR…………………………………………………………...….48 3.3.5 ELISA to Detect Anti-Avian HEV Antibody in Chickens…………………....48 3.3.6 Sequence and Phylogenetic Analyses…………………………………….49 3.3.7 Experimental Infection of Young SPF Chickens with Avian HEV Isolates Recovered from A Healthy Chicken Flock…………….………......49 3.3.8 GenBank Accession Numbers……….………………………………......50 3.4 Results………………………………………………………………...…51 3.4.1 Subclinical Infection of Chickens by Avian HEV in A Commercial Chicken Farm……………………………………………………...….51 3.4.2 Genetic Identification and Characterization of Avian HEV Isolates from A Healthy Chicken Flock……………………………..………..….51 3.4.3 Avian HEV Isolates from A Healthy Chicken Flock Is Transmissible to Young SPF Chickens under Laboratory Conditions…………...…….......52 3.4.4 The Capsid Gene of Avian HEV Isolates Recovered from Chickens with HS Syndrome in Different Geographic Regions of the United States Is Heterogeneic…...……………….………..…………………...53 3.5 Discussion……………………………………………………………….54 3.6 Acknowledgements…………………………………………………..…..56 3.7 References…………………………………………………………..…...57 3.8 Figures……………………………………………………………...…...64 3.9 Tables………………………………………………………………....…68 Chapter 4. Generation and Infectivity Titration of An Infectious Stock of Avian Hepatitis E Virus (HEV) in Chickens and Cross-Species Infection of Turkeys with Avian HEV (Published in Journal of Clinical Microbiology. Sun et al. 2004)………………………………………………………………...72 4.1 Abstract…………………………………………………………….....…72 4.2 Introduction…………………………………………………………......74 4.3 Materials and Methods……………………………………………….....75 4.3.1 Virus……………………………………………………..…………..75 4.3.2 Primer Design…………………………………………………….…..75 4.3.3 RNA Extraction and RT-PCR……………………………………….…..75 4.3.4 ELISA to Detect Anti-HEV Antibody in Chickens and Turkeys…………..….76 4.3.5 Generation of An Infectious Stock of Avian HEV……………………….…76 4.3.6 Infectivity Titration of the Avian HEV Stock in Young SPF Chickens………..77 4.3.7 Attempt to Experimentally Infect Young SPF Turkeys with Avian HEV from A Chicken……………..………………………………...….77 4.3.8 GenBank Accession Numbers……….………………………………......78 x 4.4 Results…………………………………………………………..….……78 4.4.1 Generation of An Infectious Stock of Avian HEV……………………..…...78 4.4.2 Determination of the Infectivity Titer of An Avian HEV Stock In Vivo…..…...78 4.4.3 Subclinical Infection of Young Chickens by Avian HEV in A Dose-Dependent Manner……………..…….…………………………..79 4.4.4 Cross-Species Infection of SPF Turkeys with Avian HEV from A Chicken………………………….…………………………..….….79 4.5 Discussion……………………………………..………………….……..80 4.6 Acknowledgements…………………………..…………………….….…81 4.7 References……………………………………………..…………….…..82 4.8 Figures……………………………………………………..………..…..87 4.9 Tables……………………………………………………………………88 Chapter 5. Characterization of the ORF3 Proteins of Human, Swine and Avian Hepatitis E Viruses (HEV): Identification of Antigenic CrossReactivity between Swine HEV and Human HEV but Failure to Detect the ORF3 Protein in Native Virions (To Be Submitted to Journal of Virology. Sun et al.)………...………………………………………..…..….…92 5.1 Abstract………………………………………………………..………...92 5.2 Introduction………………………………………………………..…....94 5.3 Materials and Methods……………………………………………...…..96 5.3.1 Cloning and Expression of the Truncated Avian HEV ORF2 Capsid Protein……………..…………………………………...……..96 5.3.2 Expression and Purification of ORF3 Proteins of Avian HEV and Swine HEV………………………………..……………….……..96 5.3.3 Generation of Antisera against the ORF3 Proteins of Avian, Swine and Human HEVs…….…………………….…………………...97 5.3.4 Antigenic Cross-Reactivity among the ORF3 Proteins of Avian, Swine and Human HEVs.……………………….…………………...…98 5.3.5 Purification of Avian and Swine HEV Virions………………………..…...98 5.3.6 Characterization of Virion Proteins of Avian HEV and Swine HEV…….…...99 5.3.7 ELISA to Detect Antibodies against Avian HEV ORF3 Protein in Chickens Experimentally Infected with Avian HEV……………...…...…99 5.4 Results…………………………………………………..………….…..100 5.4.1 Expression and Purification of the Truncated ORF2 Capsid Protein of Avian HEV…...…………………………………………….…..…100 5.4.2 Expression and Purification of ORF3 Proteins of Avian HEV and Swine HEV………………………………………………………......100 5.4.3 Titration of Antisera against the ORF3 Proteins of Avian, Swine and Human HEVs…………………………..…….…………….…....101 xi 5.4.4 Antigenic Cross-Reactivity among the ORF3 Proteins of Avian, Swine and Human HEVs by Western Blot Analyses……………..……......101 5.4.5 Purification and Characterization of Virion Proteins of Avian HEV and Swine HEV……..….………..………………………...……101 5.4.6 Detection of Antibodies against Avian HEV ORF3 Protein in Chickens Experimentally Infected with Avian HEV……………….…..….102 5.5 Discussion………………………………………………………...……102 5.6 Acknowledgements……………………………………………….….....104 5.7 References……………………………………………………….……..105 5.8 Figures…………………………………………………………...…….111 5.9 Tables……………………………………………………………...…...121 Chapter 6. Evidences of A Hepatitis E Virus (HEV)-Like Agent in Wild Mice in the United States and Attempt to Experimentally Infect Wistar Rats with Strains of HEVs Recovered from A Human, A Pig and A Chicken (To Be Submitted. Sun et al.)………………………………………122 6.1 Abstract……………………………………………………..…….……122 6.2 Introduction……………………………………………………..….….123 6.3 Materials and Methods……………………………………………...…124 6.3.1 Field Mouse Study………………………………..………………..…124 6.3.1.1 Trapping and Sample Collection of Field Mice in Chicken Farms……124 6.3.1.2 ELISA to Detect IgG Anti-HEV Antibodies in Field Mice……………124 6.3.1.3 RT-PCR to Detect HEV-Like Sequences from Mouse Samples……….124 6.3.1.4 Sequence Comparison and Phylogenetic Analyses…………….....…125 6.3.2 Attempts to Experimentally Infect Laboratory Mice with PCR-Positive Fecal Materials Recovered from Wild Field Mice………………….….…125 6.3.2.1 Animals…………………………………….…………….……125 6.3.2.2 Experimental Design……………………………………..…...…126 6.3.3 Attempts to Experimentally Infect Wistar Laboratory Rats with Strains of HEV Recovered from A Human, A Pig, and A Chicken……….……..…126 6.3.3.1 Viruses……………………………………………….….…….126 6.3.3.2 Experimental Inoculation of Laboratory Rats……………………....127 6.3.3.3 RT-PCR to Detect HEV RNA in Inoculated Rats……………..….….127 6.3.3.4 Generation of Rat Antiserum against the ORF2 Capsid Protein of Human HEV….………….………………………………...…128 6.3.3.5 ELISA to Detect IgG Anti-HEV Antibodies in Inoculated Rats……….129 6.4 Results……………………………………………………………….....129 6.4.1 Detection of IgG Anti-HEV Antibodies in Field Mice Caught in A Chicken Farm………………………………………………………..129 6.4.2 Detection of HEV-Like Sequences from Field Mice Caught in A xii Chicken Farm……………………………………………………......130 6.4.3 Phylogenetic Analyses of HEV-Like Sequences from Field Mice………......131 6.4.4 Failure to Transmit HEV Infection to Laboratory Mice with PCR-Positive Fecal Materials Recovered from Field Mice………...…..…131 6.4.5 Failure to Experimentally Infect Wistar Rats with Human HEV, Swine HEV or Avian HEV………………………….……………....…131 6.5 Discussion……………………………………..……………….………132 6.6 Acknowledgements………………………………..………………....…134 6.7 References…………………………………………………………...…135 6.8 Figures…………………………………………………………...…….141 6.9 Tables……………………………………………………………......…146 Chapter 7. General Conclusions and Future Research Directions………148 References…………………….……………………..………………….…….151 Chapter 8. Characterization of the Severe Acute Respiratory Syndrome (SARS) Coronavirus Nucleocapsid Protein: Identification of Common Antigenic Epitope(s) with Group I Animal Coronaviruses and Implication for SARS Diagnisis (Published in Journal of Clinical Microbiology. Sun et al. 2004)………………..………………..…...155 8.1 Abstract……………………………………………………..…….....…155 8.2 Introduction, Materials and Methods, Results and Discussion………..157 8.3 Acknowledgements………………………………..………..….…….…159 8.4 References……………………………………………..….……………160 8.5 Figures…………………………………………………….……..…….162 Curriculum Vitae………………………………………………….……..….166 xiii LIST OF FIGURES Figure 2.1 HMA analyses of swine and avian HEV isolates with respective reference viruses……………………………….……………………….…..……39 Figure 2.2 Phylogenetic analyses of swine and avian HEV sequences of targeted genomic region (s)………….………………………………………………....…40 Figure 3.1 Seroconversion to avian HEV antibody in six representative chickens from the prospective study…………………..………………………….….…….64 Figure 3.2 Phylogenetic analyses based on the nucleotide sequences of partial helicase and ORF2 capsid gene regions of avian HEV isolates and Australian chicken big liver and spleen disease virus (BLSV), as well as selected known representative strains of swine and human HEVs……….......65 Figure 3.3 Seroconversion to avian HEV antibody in six inoculated and one contact control chickens in the transmission study………….……...…....………67 Figure 4.1 Cross-species infection of 1-week-old turkeys with avian HEV from a chicken: seroconversion to avian HEV antibody in seven representative turkeys………………………………….…………………………………..….…87 Figure 5.1 Genetic map of plasmid pRSET C/AHEV ORF3……………………..……..…111 Figure 5.2 Genetic map of plasmid pRSET C/SHEV ORF3……………………...……..…112 Figure 5.3 Cloning of avian HEV ORF3 gene……………………………………...………113 Figure 5.4 Cloning of swine HEV ORF3 gene……………………………….…….………114 Figure 5.5 Expression of ORF3 proteins of avian and swine HEVs…………………..…...115 Figure 5.6 Antigenic cross-reactivity between the ORF3 proteins of avian, swine and human HEVs…………………………………………….….……….…..…117 Figure 5.7 Characterization of avian HEV virion proteins………………….……...………119 Figure 5.8 Characterization of swine HEV virion proteins………………………..……….120 Figure 6.1 Prevalence of IgG anti-HEV antibodies to ORF2 capsid proteins of avian HEV, swine HEV and human HEV in field mice (Mus musculus) trapped in a chicken farm……………...….……………………………….…....141 Figure 6.2 Phylogenetic analyses based on the nucleotide sequences of the partial helicase gene of HEV sequences from field mice, avian HEV isolates xiv and Australian chicken big liver and spleen disease virus (BLSV), as well as known representative strains of swine and human HEVs……….……..143 Figure 6.3 Detection of IgG anti-avian HEV antibody in laboratory mice experimentally inoculated with PCR-positive fecal materials recovered from field mice and with avian HEV………………….……...……..145 Figure 8.1 Genetic map of plasmid pRSET C/SARS-CoV N………………………….…..162 Figure 8.2 Cloning of the nucleocapsid (N) gene of SARS-CoV……………………..……163 Figure 8.3 Expression of SARS-CoV N protein in E. Coli……………….………….…….164 Figure 8.4 Western blot analyses of antigenic cross-reactivity of SARS-CoV N protein with polyclonal antisera of known animal coronaviruses…….……..…165 xv LIST OF TABLES Table 2.1 Field isolates of swine and avian hepatitis E viruses used in the HMA analyses and their nucleotide sequence identities with respective reference strains…...…………………………………………………….……...…42 Table 3.1 Seroconversion to IgG avian HEV antibody in healthy chickens from a normal commercial chicken farm: a prospective study………….………..….…68 Table 3.2 Pairwise sequence comparison of the helicase gene region of different avian HEV isolates, BLSV and selected known representative strains of swine and human HEVs…...………………...……………………..………..…69 Table 3.3 Pairwise sequence comparison of the ORF2 capsid gene region of different avian HEV isolates and selected known representative strains of swine and human HEVs………………………..…………………………...….70 Table 3.4 Detection of avian HEV RNA in serum and fecal samples of chickens experimentally inoculated with avian HEV isolates recovered from a healthy chicken flock………………………...……………………….…..……..71 Table 4.1 Anti-avian HEV antibody seroconversion by infectivity titration of an avian HEV stock in young SPF chickens…………………………….……....…..88 Table 4.2 Viremia and fecal virus shedding of avian HEV in young chickens experimentally inoculated with different doses of an avian HEV stock……..…..89 Table 4.3 Anti-avian HEV antibody seroconversion in turkeys experimentally inoculated with avian HEV from a chicken………………..………………...…..90 Table 4.4 Detection of avian HEV RNA in serum and fecal samples of turkeys experimentally inoculated with avian HEV from a chicken…………...…………91 Table 5.1 Seroconversion to avian HEV ORF2 and ORF3 antibodies in chickens experimentally infected with avian HEV……………………..…………....……121 Table 6.1 Time at which ninety house mice were trapped inside eight different chicken houses on a poultry farm…...………………………………….………..146 Table 6.2 Sequence comparison of HEV isolates from field mice in a chicken farm with other known isolates of avian HEV and selected representative xvi strains of swine and human HEV based on the partial helicase gene………..….147 xvii GENERAL INTRODUCTION Hepatitis E was first described as an acute enterically transmitted non-A, non-B hepatitis (ENANB) (1, 7, 16, 46, 61). It is a major public health concern in many developing countries, such as Asia, Africa and Latin America, and also occurs as sporadic cases in many industrialized countries including the United States (4, 13, 16, 22, 25, 30, 33-34, 4748, 50-51, 67, 76, 80, 89-90, 102, 104). The disease is mainly transmitted through the fecalcontaminated drinking water (4, 7). The causative agent, hepatitis E virus (HEV), is a positive sense, single-stranded, non-enveloped RNA virus with a size of about 27-34 nm (13, 19). The viral genome is about 7.2 kb with three open reading frames (ORFs) (1, 13, 19). The HEV isolates identified so far have been clustered into at least four major genotypes (19). Although the sequences of different HEV isolates are heterogeneic, they all belong to a single serotype (19, 77). HEV has recently been reclassified into the sole genus Hepevirus of the family Hepeviridae (21). The first animal strain of HEV, swine HEV, was discovered and characterized in 1997 from pigs in the United States (52). Many swine HEV isolates have subsequently been identified from pigs worldwide (26, 47, 61-62, 83, 85, 87, 95-96, 100). Swine HEV is genetically and antigenically related to the human HEV with 70-99% nucleotide sequence identity (31, 61, 95). Another animal strain of HEV, avian HEV, was identified in 2001 in the United States from chickens with hepatitis-splenomegaly (HS) syndrome (30). Avian HEV is also genetically and antigenically related to the human and swine HEVs with approximately 50% nucleotide sequence identity (31, 37). Swine HEV of genotype 3 in the United States infected non-human primates, and the U.S. strain of human HEV (also genotype 3) is able to infect SPF pigs (28, 53). Hepatitis E patients in Japan became infected after eating raw pig liver and deer meat. The sequences recovered from the patients and from the meat products were identical or near identical (83, 88, 101), indicating that at least strains of HEV in genotypes 3 and 4 can cross species barriers and infect other species (28, 53, 81, 83, 88). Thus, hepatitis E is a zoonosis (56-57). The objectives of my dissertation are to (1) assess the ability of cross-species infection of the newly identified avian HEV; (2) develop a rapid pre-sequencing assay to xviii select field isolates of HEV for future genetic studies; (3) determine the nature and prevalence of avian HEV infection in chicken flocks; (4) characterize the structural proteins of avian and swine HEVs; and (5) evaluate the potential role of rodents (mice and rats) in HEV transmission. Completion of these objectives will significantly advance our understanding of HEV epidemiology, ecology and biology. xix CHAPTER I Literature Review 1.1 Introduction Hepatitis E is an infectious viral disease that is responsible for a major proportion of the acute enterically transmitted non-A, non-B hepatitis (ENANB) (1, 7, 16, 46, 61). This disease is mainly transmitted through the fecal-contaminated drinking water (4, 7). Hepatitis E is a very important public health problem in semi-tropical countries where the hygiene is poor, such as the Southeast Asia, Africa and Latin America (4, 34, 90). Sporadic cases of acute hepatitis E have also been reported in industrialized countries, such as the United States, Japan, and many European countries (13, 16, 22, 25, 30, 33, 47-48, 50-51, 67, 76, 80, 89, 102, 104). It is believed that hepatitis E is more widely spread than previously thought (19, 49). 1.2 History Hepatitis E was not recognized until 1980 when the sera from infected patients during a large waterborne epidemic of viral hepatitis in the mid-1950s in India were tested retrospectively and found that no evidence of hepatitis A virus infection existed (1, 4, 19). The epidemiological features of the several epidemics of enterically transmitted hepatitis occurred in Europe and the United States in the past centuries and earlier this century are similar to those of hepatitis E, not hepatitis A, indicating that HEV infection may have been widespread worldwide in the past (1, 19). The first evidence for the presence of a new viral hepatitis agent was reported in 1983 (7). M. Balayan in the former Soviet Union experimentally transmitted the disease to a human volunteer by orally ingesting the fecal material from patients with enterically transmitted non-A, non-B hepatitis. Viral particles were observed from the feces of the volunteer by immune electron microscopy. The disease was also successfully transmitted to cynomolgus monkeys (3). The partial genome of this virus was cloned in 1990 and 1 fully sequenced soon after (72, 86, 91). The causative agent of this disease was renamed as hepatitis E virus (HEV) (68). 1.3 Virology HEV is an non-enveloped, positive-sense, single-stranded RNA virus with a size of 27-34 nm in diameter (13, 19). The viral genome is about 7.2 kb, consisting of a short 5' nontranslated region (27-35 nucleotides) followed by three partially overlapping open reading frames (ORFs), a 3' nontranslated region (about 65-74 nucleotides), and a poly A tract (1, 13, 19). It was believed that ORF1 encoded nonstructural proteins, such as the putative methyltransferase, protease, X domain, helicase, and RNA-dependent RNA polymerase. ORF2 was postulated to encode a major capsid protein, and ORF3 appeared to encode a small phosphoprotein with only 123 amino acids, which is associated with the cytoskeleton (103). The virus is not able to grow efficiently in cell culture (19). HEV has recently been reclassified as the sole member of the genus Hepevirus in the family Hepeviridae (21). The first animal strain of HEV, swine HEV, was identified from pigs in the United States in 1997 (52). Subsequently, numerous swine HEV isolates have been identified worldwide (26, 47, 61-62, 83, 85, 87, 95, 96, 100). Swine HEV is genetically and antigenically related to human strains of HEV, with approximately 70-100% nucleotide sequence identity (31, 61, 83, 95). Another animal strain of HEV, avian HEV, was identified from chickens with hepatitis-splenomegaly (HS) syndrome in the U.S. in 2001 (30). A recent report showed that avian HEV was also present in chickens in Canada (2). Avian HEV is genetically and antigenically related to human and swine strains of HEV (31). It shares about 50% nucleotide sequence identity over the entire genome, 48-51% identity in ORF1, 46-48% identity in ORF2, but only 29-34% identity in ORF3 with the human and swine HEV strains (37). At least four major genotypes of HEV have been identified, with the representative strain of Sar-55 from Pakistan and isolates from Asian countries in genotype 1, a single strain of HEV from Mexico in genotype 2, human and swine HEV strains in the U.S. and Europe in genotype 3, and some recent variant isolates from China, Taiwan and Japan in genotype 4 (19). Although the sequences of HEV strains are very heterogeneic, it seems 2 that all HEV isolates studied to date belong to a single serotype (19, 77). It has been reported that coinfection with two different strains or even two different genotypes of HEV in one individual existed, and quasispecies of HEV infection was also suggested (27, 82, 84, 87). 1.4 Molecular Characteristics Due to the lack of a reliable cell culture system to support HEV replication, information about its molecular biology was mainly obtained from recombinant technologies (75). Various genes or genomic regions of HEV have been cloned and expressed in different expression systems. ORF1 gene encodes the largest protein with motifs characteristic of a methyltransferase, a papain-like protease, a helicase and an RNA-dependent RNA polymerase. ORF1 has been reported to accumulate as an unprocessed protein of 186 kDa, and then processed into either 78 and 107 kDa, or 35 and 40 kDa protein products (19, 39). Since the 5' nontranslated region (NTR) of the HEV genome is only about 27 nucleotides long, it is likely that translation took place through a 'cap' initiation mechanism (18, 20). Indeed, it has been demonstrated that the genome of HEV is capped at its 5’-end (40). ORF2 gene encodes a putative capsid protein of approximately 660 amino acids. It is believed to be the major protein in the virion (19). The full-length recombinant ORF2 protein was expressed as a 72 kDa protein in cultured cells and quickly cleaved by non-viral proteolytic enzymes into several subunits with the sizes of 53 kDa, 55 kDa and 63 kDa (19, 39). ORF3 gene of HEV encodes a small protein, with only 123 amino acids for most HEV strains (19). It is not clear if the ORF3 protein is a component of the HEV virion, or a nonstructural protein. Recombinant ORF3 protein expressed in eukaryotic cells accumulates in the cytoplasm and separates with the cytoskeleton (103). It is postulated that ORF3 may be a viral regulatory protein involved in modulation of cell signaling. Yeast two-hybrid and co-immunoprecipitation assays showed that recombinant ORF3 protein also interacts with ORF2 protein (94). It bound preferentially to the nonglycosylaed form of ORF2 protein and only to the full-length protein, which supported the postulation that ORF3 may play a role in HEV assembly (94). 3 1.5 Animal Models A variety of cell lines have been tested, and an efficient cell culture system has not been found yet for HEV propagation. HEV was first experimentally transmitted to a macaque, since then macaques are the species of choice for HEV studies (19, 70). Rhesus and cynomolgus macaques have been used for evaluating candidate vaccines as well. Since clinical signs of hepatitis E are dose-dependent in these animal models and production of disease requires challenge doses 1,000-fold higher than the amount for infectivity, it is difficult for evaluating vaccine efficacy (70). However, with the identification of avian and swine strains of HEVs, SPF chickens and pigs can now be used as animal models to study virus replication and pathogenesis of this disease (30, 52). Although humans normally get infected through ingestion of contaminated water or food under natural conditions, and infection was experimentally reproduced by the natural oral route of inoculation in non-human primates and in chickens, the oral route is not efficient to infect animals (3, 12, 19). Therefore, viruses are usually inoculated intravenously in animal studies. The pathogenesis of hepatitis E is not well known, but it is believed that HEV first replicates in the GI tract before reaching the liver (97). Viruses accumulate in the bile to a very high concentration and are subsequently shed in the feces (19). It was reported that, in pigs, swine HEV and human HEV replicated in liver, spleen, lymph nodes, and intestinal tract. Viremia and fecal virus shedding are observed before liver abnormalities occur (28, 97). Scientists in the former Soviet Union and Thailand reportedly transmitted laboratory mice and Wista rats with a human strain of HEV (42, 48). However, their results could not be independently confirmed (J. C. Wu, personal communication). 1.6 Epidemiology and Seroprevalence 1.6.1 Epidemiological Features and Transmission Routes HEV often causes epidemic and sporadic cases of acute hepatitis in subtropical regions in Asia, Africa, the Middle East, and Central America (1, 99). Many of these outbreaks involved large numbers of cases, such as the one in Xinjiang, China in 1986- 4 1988 with about 100,000 cases (1, 19, 49). HEV also causes sporadic cases in areas previously thought to be non-endemic such as the United States, Japan and some European countries (13, 16, 22, 30, 33, 47-48, 50-51, 67, 76, 80, 89, 104). It was reported that the seroprevalence is much lower than anticipated (3-26%) in endemic regions, however is higher than expected (1-3%) in non-endemic areas (19). Fecal-oral route is believed to be the major mode of transmission (3, 5, 10). In HEV-endemic regions, outbreaks are mainly associated with fecal contaminated drinking water after heavy floods (5). Food-borne epidemics (via consumption of raw or undercooked shellfish, meat products) have also been documented and believed to be the source of sporadic cases in some endemic areas (1). The occurrence of sporadic HEV infections in humans may maintain transmission during inter-epidemic periods. Unlike HAV, person-to-person transmission of HEV is uncommon (19). In non-endemic regions, sporadic cases were reported among travelers to endemic regions (44, 64). Consumptions of raw or undercooked shellfish, pig liver, deer meat in Japan were linked to cases of hepatitis E (83, 88, 101). Although only sporadic cases of hepatitis E were reported in the U.S. and other industrialized countries, anti-HEV antibodies have been found in a significant proportion (up to 28% in some areas) of healthy individuals (58, 89, 98). Subclinical infection of humans with swine HEV or other animal strains of HEV may explain the relatively high prevalence of anti-HEV antibodies in healthy individuals in non-endemic areas (58, 98). Regardless of whether or not HEV is endemic in the respective human population, hepatitis E is enzootic in pigs worldwide (19). 1.6.2 Cross-Species Infections Thus far, antibodies to HEV have been detected in a variety of animal species, including monkeys, pigs, chickens, sheep, goats, cattle, dogs, cats, rats, and mice (6-7, 14, 24, 30, 32, 41-42, 46, 52, 55, 63, 78, 80, 90, 96, 98, 100, 102). HEV sequences have been identified from domestic and miniature pigs, wild boars, chickens, and deer (2, 30, 33, 47, 52, 62, 66, 80, 83, 87-88, 95, 96). Genotype 3 HEV can infect SPF pigs and genotype 3 swine HEV infected non-human primates (28, 53). Avian HEV is able to infect SPF turkeys (81). Hepatitis E patients in Japan became infected with HEV after 5 consuming raw pig liver and deer meat (83, 88, 101). The sequences identified from the patients were identical or near identical to the viruses recovered from raw meats (83, 88). These data indicated that at least some strains of HEV, such as genotypes 3 and 4 strains, are able to cross species barriers and infect other species (28, 53, 81, 83, 88). Therefore, hepatitis E is a zoonotic disease (56-57). 1.6.3 Animal Reservoirs Swine veterinarians and other pig handlers have been found to have a higher risk of seropositivity to HEV antibodies, suggesting that pigs may serve as an animal reservoir (58, 98). Recent reports from Japan showed that deer and wild boars could also be animal reservoirs for HEV (78, 83, 88). Wild rodents may also act as the animal reservoir, and high prevalence of anti-HEV has been reported in rats (7, 24, 32, 41, 98). However, so far the virus responsible for the seropositivity in rats has not yet been isolated (19). We previously showed that avian HEV can not be transmitted to nonhuman primates, and thus chickens might not be a reservoir for HEV infection in humans (37). 1.7 Clinical Features The clinical symptoms of hepatitis E are similar to hepatitis A, but more severe (1, 19). The incubation period of hepatitis E ranges from 2 to 8 weeks. The characteristic signs include jaundice, anorexia, hepatomegaly, abdominal pain and tenderness, nausea, vomiting and fever (13). The disease may vary in severity from subclinical infection to fulminant hepatitis (19). Symptomatic HEV infection is generally seen in middle-aged adults. Children may develop severe forms of disease, such as acute liver failure by coinfecting with hepatitis A virus (HAV) (99). Viremia and fecal virus shedding occur during the incubation period and early acute phase of disease (1). Presence of HEV antigens in the liver usually coincides with the appearance of viremia and fecal virus shedding (19). Hepatitis E is a self-limiting disease usually with complete recovery (99). A fulminant form of hepatitis may occasionally develop, with a low mortality rate of about 6 1% in the general population (1, 19). However, a mortality rate of up to 20% can be reached during the 3rd trimester of pregnancy in fulminant hepatitis cases (29, 38, 45, 69). It is hypothesized that HEV might precipitate eclampsia and cause increased mortality in pregnant women by directly or indirectly affecting the kidneys (9, 99). HEV infection does not go into chronicity (19, 49, 99). 1.8 Pathogenesis Due to the lack of a reliable and efficient cell culture system and a practical animal model, an understanding of HEV associated pathology has mainly been obtained through studies in non-human primates, and human volunteers (1). In monkeys, HEV itself does not cause liver damage. However, the immune response induced during viral replication may cause liver damage (1, 19, 70). It was believed that seroconversion to HEV cleared the virus from feces and blood and coordinated the disease resolution (1, 99). In general, both IgM and IgG anti-HEV develop at the time of disease onset (15). IgM anti-HEV usually precedes the IgG anti-HEV by a few days and IgM anti-HEV titer declines rapidly during early convalescent phase (1, 19, 99). IgG anti-HEV lasts much longer and have been shown to persist for over 14 years in about 50% of the patients and provide protection against subsequent infections (15, 43, 49). Human volunteer studies showed that liver enzymes elevated 4-5 weeks after oral ingestion of HEV and persisted for 1-3 months. Fecal virus shedding started around 4 weeks after oral ingestion and lasted for about 2 weeks (10). Viruses could be detected in bile, liver and feces prior to the onset of liver disease (1, 19, 99). Histopathologic lesions in liver, include both cholestatic and standard acute viral hepatitis (1). HEV antigen can be detected in the cytoplasm of infected hepatocytes starting 10 days after intravenous inoculation and persisting for about 3 weeks. A cholestatic type of hepatitis was found in the majority of cases in studies of patients involved in several HEV outbreaks (1, 19). Severe acute hepatitis with hepatic necrosis was observed in some 7 patients, especially in severe or fulminant cases (49, 99). No evidence of chronic hepatitis has been detected among patients during the clinical follow-up after acute hepatitis (49, 69). 1.9 Diagnosis Hepatitis E can not be clinically distinguished from other types of acute viral hepatitis (69). If outbreaks of waterborne hepatitis occurred in developing countries, especially if the disease is more severe in pregnant women, or if hepatitis A is not the causative agent, hepatitis E should be considered (99). HEV ORF2 and ORF3 proteins have been expressed in various recombinant systems, including Escherichia coli and baculovirus-infected insect cells, and these recombinant proteins have been widely used for diagnostic tests (75, 92). By using recombinant proteins or synthetic peptides, ELISA and Western blot have been used to detect IgG anti-HEV antibodies in serum (11, 23, 92). IgM assays have also been developed to differentiate acute infection from past infection (19). RT-PCR is used to detect HEV RNA in serum, stool and bile samples (93). Immunofluorescent antibody assay can be used to detect HEV antigen in liver and other tissues (11). Immune electron microscopy can be used to detect viral particles in feces and bile samples (10, 11). 1.10 Prevention and Vaccines Passive immunization with immunoglobulin (Ig) is not available at this time (17, 49). Cynomolgus macaques injected with convalescent phase plasma from an infected animal provided protection against clinical disease after challenge, but not against infection (1, 99). In non-endemic areas, Ig prepared from plasma of donors does not prevent clinical disease due to the lack of sufficient levels of protective antibody in the preparation (1, 17, 19, 69). Prevention is the most effective measure against hepatitis E. Good personal hygiene, high quality of public water system and proper disposal of sanitary waste are 8 effective measures to prevent HEV infection (1, 49). Common hygiene practices should be taken to prevent hepatitis E if travelling to endemic regions (99). Because a cell culture system to efficiently propagate HEV is lacking, it is not possible to develop an attenuated or killed vaccine at this time (19). However, the recombinant antigen vaccine is very promising, and may provide short-term protection (17, 71, 105). 1.11 Swine Hepatitis E Virus (Swine HEV) The first animal strain of HEV, swine HEV, was discovered in the United States in 1997 (52). Since then, many isolates of swine HEV have been identified from pigs in many other countries and the infection is widespread in pig populations (26, 47, 62, 66, 83, 87, 96). Anti-HEV antibodies are highly prevalent in pigs worldwide (7, 14, 60, 78, 96, 98, 100). Pigs usually get infected between the ages of 2 and 3 months old (39). However, there is no gross lesion in infected pigs during necropsy (28, 54). The genome organization of swine HEV is very similar to that of human HEV (52). Swine HEV is genetically and antigenically close-related to human HEV (61, 85, 95). They share approximately 70-100% nucleotide sequence identity with each other (31, 61, 83, 95). Like human HEV, swine HEV isolates identified to date belong to at least two different genotypes (60). In general, the HEV isolates identified from swine and humans in the same geographic area belong to the same genotype (8, 22, 33, 52, 76, 82, 84). The sequences of swine HEV isolates are highly heterogeneic (35, 79). However, only one serotype of swine HEV has been identified so far regardless of the geographic regions (59-60). It was reported that the genotype 3 of the U.S. swine HEV is able to experimentally infect nonhuman primates, and the U.S. human HEV of genotype 3 can infect SPF pigs (28, 53). HEV contaminated raw pig liver or boar meat can transmit hepatitis E to humans in Japan through food consumption (83, 88, 101). The infection with swine HEV in some nonendemic regions may be responsible for the high prevalence of anti-HEV antibody in humans (19, 60). The demonstrated ability of cross-species infection of HEV between pigs and humans indicates that HEV is a zoonosis, and pigs are animal reservoirs (57, 60). The zoonotic risk of swine HEV raises potential concerns 9 of zoonosis or xenozoonosis for using pigs as organ donors in xenotransplantation (59). On the other hand, pigs can serve as an animal model to study the replication and pathogenesis of HEV (52). 1.12 Avian Hepatitis E Virus (Avian HEV) Hepatitis-splenomegaly (HS) syndrome was first reported in Canada in 1991 and has since been recognized in United States (73-74). The characteristics of this disease include increased mortality in young laying birds of 30-72 weeks of age with 20% drop in egg production (73). The causative agent of this disease was not known. However, a study conducted in Vietnam showed that human HEV antibody was prevalent in 44% of chickens, indicating that a HEV or a HEV-like agent may infect chickens (90). In 2001, avian HEV, the second animal strain of HEV, was isolated in the United States from chickens with HS syndrome (30). Avian HEV infection is widespread in chicken flocks in the United States (30, 36, 80). Recently, avian HEV was also isolated from commercial chickens in Canada (2). Like swine and human HEVs, avian HEV isolates from chickens in different geographic regions are also heterogeneic (36, 79-80). Subclinical infection was recognized and avian HEV isolates were also identified from apparently healthy chickens in the United States (80). The genome organization of avian HEV is similar to that of swine and human HEVs (30). Avian HEV is genetically related to swine and human HEV strains, sharing about 50% nucleotide sequence identity over the complete genome (37). Avian HEV is also antigenically related to swine and human HEV strains (31). Avian HEV is related to, but distinct from the Australian chicken big liver and spleen disease virus (BLSV), with about 80% nucleotide sequence identity (31, 65). Phylogenetic analyses indicate that avian HEV isolates identified in the United States are clustered into a new genotype (36). Avian HEV has been shown to be able to experimentally infect turkeys, however, attempts to experimentally transmit avian HEV to nonhuman primates have not been successful (37, 81). Like swine HEV, avian HEV could potentially serve as an animal model for human HEV study as well (30, 59-60). 10 1.13 References 1. Aggarwal, R., and K. Krawczynski. 2000. Hepatitis E: An overview and recent advances in clinical and laboratory research. J. Gastroenterol. Hepatol. 15:9-20. 2. Agunos, A. C., B. Hunter, D. Yoo, B. Binnington, S. Youssef, and D. L. Ran. Avian Hepatitis E virus isolated in commercial layers submitted to the University of Guelph (Only the GenBank accession no. of the sequences are available: AY671802, AY671803, AY671804 and AY671805). 3. Andzhaparidze, A. G., M. S. Balaian, A. P. Savinov, D. M. Braginskii, and V. F. Poleshchuk. 1986. Reproduction in monkeys of non-A, non-B hepatitis transmitted via the fecal and oral routes (in Russian). Vopr Virusol. 31:73-81. 4. Arankalle, V. A., M. O. Favorov, M. S. Chadha, D. M. Phule, and K. Banerjee. 1993. Rhesus monkeys infected with hepatitis E virus (HEV) from the former USSR are immune to subsequent challenge with an Indian strain of HEV. Acta. Virol. 37:515-518. 5. Arankalle, V. A., M. S. Chadha, S. A. Tsarev, S. U. Emerson, A. R. Risbud, K. Banerjee, and R. H. Purcell. 1994. Seroepidemiology of water-borne hepatitis in India and evidence for a third enterically-transmitted hepatitis agent. Proc. Natl. Acad. Sci. USA 91:3428-3432. 6. Arankalle, V. A., M. K. Goverdhan, and K. Banerjee. 1994. Antidodies against hepatitis E virus in old world mondays. J. Viral Hepatitis 1:125-129. 7. Arankalle, V. A., M. V. Joshi, A. M. Kulkarni, S. S. Gandhe, L. P. Chobe, S. S. Rautmare, A. C. Mishra., and V. S. Padbidri. 2001. Prevalence of anti-hepatitis E virus antibodies in different Indian animal species. J. Viral Hepatitis 8:223-227. 8. Arankalle V. A., L. P. Chobe, A. M. Walimbe, P. N. Yergolkar, and G. P. Jacob. 2003. Swine HEV infection in south India and phylogenetic analysis (1985-1999). J. Med. Virol. 69:391-396. 9. Asher, L. V. S., et. al. 1997. Acute tubular necrosis in kidneys of monkeys infected with hepatitis E virus, p. 331-333. In: Rizzetto M., Purcell R. H., Gerin J. L., and Verme G., eds. Viral hepatitis and liver disease. Turin, Minerva Medica. 11 10. Balayan, M. S., A. G. Andjaparidze, S. S. Savinskaya, E. S. Ketiladze, D. M. Braginsky, A. P. Savinov, and V. F. Poleshchuk. 1983. Evidence for a virus in nonA, non-B hepatitis transmitted via fecal-oral route. Intervirology 20:23-31. 11. Balayan, M. S. 1993. Hepatitis E virus infection in Europe: regional situation regarding laboratory diagnosis and epidemiology. Clin. Diagn. Virol. 1:1-9. 12. Billam, P., F. F. Huang, Z. F. Sun, D. K. Guenette, F. W. Pierson, R. B. Duncan, F. Elvinger, T. E. Toth, and X. J. Meng. 2005. Systematic pathogenesis and replication of a strain of the hepatitis E virus (HEV) in specific-pathogen-free adult chickens. J. Virol. 79(6) (March issue, In Press). 13. Bradley, D. W. 1992. Hepatitis E: epidemiology, etiology and molecular biology. Rev. Med. Virol. 2:19-28. 14. Clayson, E. T., K. S. Innis, S. Myint, S. Narupiti, D. W. Vaughn, S. Giri, P. Ranabhat, and M. P. Shrestha. 1995. Detection of hepatitis E virus infections among domestic swine in the Kathmandu Valley of Nepal. Am. J. Trop. Med. Hyg. 53:228232. 15. Clayson, E, T., K. S. Myint, R. Snitbhan, D. W. Vaughn, B. L. Innis, L. Chan, P. Cheung, and M. P. Shrestha. 1995. Viremia, fecal shedding, and IgM and IgG responses in patients with hepatitis E. J. Infect. Dis. 172:927-933. 16. Clemente-Casares, P., S. Pina, M. Buti, R. Jardi, M. Margarita, S. Bofill-Mas, and R. Girones. 2003. Hepatitis E virus epidemiology in industrialized countries. Emerg. Infect. Dis. 9:448-454. 17. Emerson, S. U., and R. H. Purcell. 2001. Recombinant vaccines for hepatitis E. Trends Mol. Med. 7:462-466. 18. Emerson, S. U., M. Zhang, X. J. Meng, H. Nguyen, M. St Claire, S. Govindarajan, Y. K. Huang, and R. H. Purcell. 2001. Recombinant hepatitis E virus genomes infectious for primates: importance of capping and discovery of a cis-reactive element. Proc. Natl. Acad. Sci. USA. 98:15270-15275. 19. Emerson, S. U., and R. H. Purcell. 2003. Hepatitis E virus. Rev. Med. Virol. 13:145154. 12 20. Emerson, S. U., H. Nguyen, J. Graff, D. A. Stephany, A. Brockington, and R. H. Purcell. 2004. In vitro replication of hepatitis E virus (HEV) genomes and of an HEV replicon expressing green fluorescent protein. J. Virol. 78:4838-4846. 21. Emerson, S. U., D. Anderson, A. Arankalle, X. -J. Meng, M. Purdy, G. G. Schlauder, and S. A. Tsarev. 2004. Hepevirus, p. 851-855. In C.M. Fauquet, M. A. Mayo, J. Maniloff, U. Desselberger, and L.A. Ball. (ed.), Virus Taxonomy, VIIIth Report of the ICTV, Elsevier/Academic Press, London. 22. Erker, J. C., S. M. Desai, G. G. Schlauder, G. J. Dawson, and I. K. Mushahwar. 1999. A hepatitis E virus variant from the United States: molecular characterization and transmission in cynomolgus macaques. J. Gen. Virol. 80:681-690. 23. Favorov, M. O., H. A. Fields, M. A. Purdy, T. L. Yashina, A. G. Aleksandrov, M. J. Alter, D. M. Yarasheva, D. W. Bradley, and H. S. Margolis. 1992. Serologic identification of hepatitis E virus infections in epidemic and endemic settings. J. Med. Virol. 36:246-250. 24. Favorov, M. O., M. Y. Kosoy, S. A. Tsarev, J. E. Childs, and H. S. Margolis. 2000. Prevalence of antibody to hepatitis E virus among rodents in the United States. J. Infect. Dis. 181:449-455. 25. Fukuda, S., J. Sunaga, N. Saito, K. Fujimura, Y. Itoh, M. Sasaki, F. Tsuda, M. Takahashi, T. Nishizawa, and H. Okamoto. 2004. Prevalence of antibodies to hepatitis E virus among Japanese blood donors: identification of three blood donors infected with a genotype 3 hepatitis E virus. J Med Virol. 73:554-561. 26. Garkavenko, O., A. Obriadina, J. Meng, D. A. Anderson, H. J. Benard, B. A. Schroeder, Y. E. Khudaakov, H. A. Fields, and M . C. Croxson. 2001. Detection and characterization of swine hepatitis E virus in New Zealand. J. Med. Virol. 65:525529. 27. Grandadam, M., S. Tebbal, M. Caron, M. Siriwardana, B. Larouze, J. L. Koeck, Y. Buisson, V. Enouf, and E. Nicand. 2004. Evidence for hepatitis E virus quasispecies. J. Gen. Virol. 85:3189-3194. 28. Halbur, P. G., C. Kasorndorkbua, C. Gilbert, D. Guenette, M. B. Potters, R. H. Purcell, S. U. Emerson, T. E. Toth, and X. J. Meng. 2001. Comparative 13 pathogenesis of infection of pigs with hepatitis E viruses recovered from a pig and a human. J. Clin. Microbiol. 39:918-923. 29. Hamid, S. S., S. M. Jafri, H. Khan, H. Shah, Z. Abbas, and H. Fields. 1996. Fulminant hepatic failure in pregnant women: acute fatty liver or acute viral hepatitis? J. Hepatol. 25:20-27. 30. Haqshenas, G., H. L. Shivaprasad, P. R. Woolcock, D. H. Read, and X. J. Meng. 2001. Genetic identification and characterization of a novel virus related to human hepatitis E virus from chickens with hepatitis-splenomegaly syndrome in the United States. J. Gen. Virol. 82:2449-2462. 31. Haqshenas, G., F. F. Huang, M. Fenaux, D. K. Guenette, F. W. Pierson, C. T. Larsen, H. L. Shivaprasad, T. E. Toth, and X. J. Meng. 2002. The putative capsid protein of the newly identified avian hepatitis E virus shares antigenic epitopes with that of swine and human hepatitis E viruses and the chicken big liver and spleen disease virus. J. Gen. Virol. 83:2201-2209. 32. Hirano, K., X. Ding, T. C. Li, N. Takeda, H. Kawabata, N. Koizumi, T. Kadosaka, I. Goto, T. Masuzawa, M. Nakamura, K. Taira, T. Kuroki, T. Tanikawa, H. Watanabe, K. Abe. 2003. Evidence for widespread infection of hepatitis E virus among wild rats in Japan. Hepatol. Res. 27:1-5. 33. Hsieh, S. Y., P. Y. Yang, Y. P. Ho, C. M. Chu, Y. F. Liaw. 1998. Identification of a novel strain of hepatitis E virus responsible for sporadic acute hepatitis in Taiwan. J. Med. Virol. 55:300-304. 34. Huang, C. C., D. Nguyen, J. Fernandez, K. Y. Yun, K. E. Fry, D. W. Bradley, A. W. Tam, G. R. Reyes. 1992. Molecular cloning and sequencing of the Mexico isolate of hepatitis E virus (HEV). Virology 191:550-558. 35. Huang, F. F., G. Haqshenas, D. K. Guenette, P. G. Halbur, S. Schommer, F. W. Pierson, T. E. Toth, and X. J. Meng. 2002. Detection by RT-PCR and genetic characterization of field isolates of swine hepatitis E virus from pigs in different geographic regions of the United States. J. Clin. Microbiol. 40:1326-1332. 36. Huang, F. F., G. Haqshenas, H. L. Shivaprasad, D. K. Guenette, P. R. Woolcock, C. T. Larsen, F. W. Pierson, F. Elvinger, T. E. Toth, and X. J. Meng. 2002. 14 Heterogeneity and seroprevalence of the newly identified avian hepatitis E virus from chickens in the United States. J. Clin. Microbiol. 40:4197-4202. 37. Huang, F. F., Z. F. Sun, S. U. Emerson, R. H. Purcell, H. L. Shivaprasad, F. W. Pierson, T. E. Toth, and X. J. Meng. 2004. Determination and analysis of the complete genomic sequence of avian hepatitis E virus (avian HEV) and attempts to infect rhesus monkeys with avian HEV. J. Gen. Virol. 85:1609-1618. 38. Hussaini, S. H., S. J. Skidmore, P. Richardson, L. M. Sherratt, B. T. Cooper, and J. G. O’Grady. 1997. Severe hepatitis E infection during pregnancy. J. Viral. Hepat. 4:51-54. 39. Jameel, S., M. Zafrullah, H. Ozdener, and S. K. Panda. 1996. Expression in animal cells and characterization of the hepatitis E virus structural proteins. J. Virol. 70:207216. 40. Kabrane-Lazizi, Y., X. J. Meng, R. H. Purcell, and S. U. Emerson. 1999. Evidence that the genomic RNA of hepatitis E virus is capped. J. Virol. 73:8848-50. 41. Kabrane-Lazizi, Y., J. B. Fine, J. Elm, G. E. Glass, H. Higa, A. Diwan, C. J. Gibbs Jr., X. J. Meng, S. U. Emerson, and R. H. Purcell. 1999. Evidence for widespread infection of wild rats with hepatitis E virus in the United States. Am. J. Trop. Med. Hyg. 61:331-335. 42. Karetnyi, Y. V., D. I. Dzhumalieva, R. K. Usmanov, I. P. Titova, Y. A. Lituak, and M. S. Balayan. 1993. Possible involvement of rodents in the spread of hepatitis E (in Russian). J. Microbiol. Epidemiol. Immunol. 41:52-56. 43. Khuroo, M. S., S. Kamili, M. Y. Dar, R. Moecklii, and S. Jameel. 1993. Hepatitis E and long-term antibody status. Lancet 341:1355. 44. Koizumi, Y., N. Isoda, Y. Sato, T. Iwaki, K. Ono, K. Ido, K. Sugano, M. Takahashi, T. Nishizawa, and H. Okamoto. 2004. Infection of a Japanese patient by genotype 4 hepatitis E virus while traveling in Vietnam. J. Clin. Microbiol. 42:38833885. 45. Kumar, A., M. Beniwal, P. Kar, J. B. Sharma, and N. S. Murthy. 2004. Hepatitis E in pregnancy. Int. J. Gynaecol. Obstet. 85:240-244. 46. Kuno, A., K. Ido, N. Isoda, Y. Satoh, K. Ono, S. Satoh, H. Inamori, K. Sugano, N. Kanai, T. Nishizawa, H. Okamoto. 2003. Sporadic acute hepatitis E of a 47-year-old 15 man whose pet cat was positive for antibody to hepatitis E virus. Hepatol. Res. 26:237-242. 47. Lu, L., J. Drobeniuc, N. Kobylnikov, R. K. Usmanov, B. H. Robertson, M. O. Favorov, and H. S. Margolis. 2004. Complete sequence of a Kyrgyzstan swine hepatitis E virus (HEV) isolated from a piglet thought to be experimentally infected with human HEV. J. Med. Virol. 74:556-562. 48. Maneerat, Y., E. T. Clayson, K. S. A. Myint, G. D. Young, and B. L Innis. 1996. Experimental infection of the laboratory rats with the hepatitis E virus. J. Med. Virol. 48:121-128. 49. Mast, E. E., and K. Krawczynski. 1996. Hepatitis E: An overview. Annu. Rev. Med. 47:257-266. 50. Mast, E. E., I. K. Kuramoto, M. O. Favorov, V. R. Schoening, B. T. Burkholder, C. N. Shapiro, and P. V. Holland. 1997. Prevalence of and risk factors for antibody to hepatitis E virus seroreactivity among blood donors in Northern California. J. Infect. Dis. 176:34-40. 51. McCrudden, R., S. O’Connell, T. Farrant, S. Beaton, J. P. Iredale, and D. Fine. 2000. Sporadic acute hepatitis E in the United Kingdom: an underdiagnosed phenomenon? Gut 46:732-733. 52. Meng, X. J., R. H. Purcell, P. G. Halbur, J. R. Lehman, D. M. Webb, T. S. Tsareva, J. S. Haynes, B. J. Thacker, and S. U. Emerson. 1997. A novel virus in swine is closely related to the human hepatitis E virus. Proc. Natl. Acad. Sci. USA 94:9860-9865. 53. Meng, X. J., P. G. Halhur, M. S. Shapiro, S. Govindarajan, J. D. Bruna, I. K. Mushahwar, R. H. Purcell, S. U. Emerson. 1998. Genetic and experimental evidence for cross-species infection by the swine hepatitis E virus. J. Virol. 72:97149721. 54. Meng, X. J., P. G. Halbur, J. S. Haynes, T. S. Tsareva, J. D. Bruna, R. L. Royer, R. H. Purcell, and S. U. Emerson. 1998. Experimental infection of pigs with the newly identified swine hepatitis E virus (swine HEV), but not with human strains of HEV. Arch. Virol. 143:1405-1415. 16 55. Meng, X. J., S. Dea, R. E. Engle, R. Friendship, Y. S. Lyoo, T. Sirinnarumitr, K. Urairong, D. Wang, D. Wong, D. Yoo, Y. Zhang, R. H. Purcell, and S. U. Emerson. 1999. Prevalence of antibodies to the hepatitis E virus in pigs from countries where hepatitis E is common or is rare in the human population. J. Med. Virol. 58:297-302. 56. Meng, X. J. 2000. Zoonotic and xenozoonotic risks of hepatitis E virus. Infect. Dis. Rev. 2:35-41. 57. Meng, X. J. 2000. Novel strains of hepatitis E virus identified from humans and other animal species: Is hepatitis E a zoonosis? J. Hepatol. 33:842-845. 58. Meng, X. J., B. Wiseman, F. Elvinger, D. K. Guenette, T. E. Toth, R. E. Engle, S. U. Emerson, and R. H. Purcell. 2002. Prevalence of antibodies to the hepatitis E virus in veterinarians working with swine and in normal blood donors in the United States and other countries. J. Clin. Microbiol. 40:117-122. 59. Meng, X. J. 2003. Swine hepatitis E virus: cross-species infection and risk in xenotransplantation. Curr. Topics Microbiol. Immunol. 278:185-216. 60. Meng, X. J. 2004. Hepatitis E as a zoonotic disease, p. In H. Thomas, A. Zuckermann, and S. Lemon. (ed.), Viral hepatitis, 3rd ed., Section VII, Chapter 42. Blackwell Publishing Ltd, Oxford, U.K. 61. Nishizawa, T., M. Takahashi, H. Mizuo, H. Miyajima, Y. Gotanda, and H. Okamoto. 2003. Characterization of Japanese swine and human hepatitis E virus isolates of genotype IV with 99% identity over the entire genome. J. Gen. Virol. 84:1245-1251. 62. Okamoto, H., M. Takahashi, T. Nishizawa, K. Fukai, U. Muramatsu, and A. Yoshikawa. 2001. Analysis of the complete genome of indigenous swine hepatitis E virus isolated in Japan. Biochem. Biophys. Res. Commun. 289:929-936. 63. Okamoto, H., M. Takahashi, T. Nishizawa, R. Usui, and E. Kobayashi. 2004. Presence of antibodies to hepatitis E virus in Japanese pet cats. Infection 32:57-58. 64. Palmovic, D., Lukas D., Vince A., Kurelac I. 2002. Hepatitis E in tourists to India (in Croatian). Lijec Vjesn. 124:313-314. 17 65. Payne, C. J., T. M. Ellis, S. L. Plant, A. R. Gregory, and G. E. Wilcox. 1999. Sequence data suggests big liver and spleen disease virus (BLSV) is genetically related to hepatitis E virus. Vet Microbiol. 68:119-125. 66. Pei, Y., and D. Yoo. 2002. Genetic characterization and sequence heterogeneity of a canadian isolate of swine hepatitis E virus. J Clin Microbiol. 40:4021-4029. 67. Pina, S., M. Buti, M. Cotrina, J. Piella, and R. Girones. 2000. HEV identified in serum from humans with acute hepatitis and in sewage of animal origin in Spain. J. Hepatol. 33:826-833. 68. Purcell, R. H., and J. R. Ticehurst. 1988. Enterically transmitted non-A, non-B hepatitis: Epidemiology and clinical characteristics, p. 131-137. In: Zuckermann A. J., ed. Viral Hepatitis and Liver Disease. New York: Alan R Liss. 69. Purcell, R. H. 1996. Hepatitis E virus, p. 2831-2843. In B. N. Fields, D. M. Knipe, and P. M. Howley, et al. (ed.), Fields virology, 3rd ed., vol 2. Lippincott-Raven Publishers, Philadelphia, Pa. 70. Purcell, R. H., and S. U. Emerson. 2001. Animal models of hepatitis A and E. Instit. Lab. Animal. Res. J. 42:161-177. 71. Purcell, R. H., H. Nguyen, M. Shapiro, R. E. Engle, S. Govindarajan, W. C. Blackwelder, D. C. Wong, J-P. Prieels, and S. U. Emerson. 2003. Pre-clinical immunogenicity and efficacy trial of a recombinant hepatitis E vaccine. Vaccine 21:2607-2615. 72. Reyes, G. R., M. A. Purdy, J. P. Kim, K. C. Luk, L. M. Young, K. E. Fry, and D. W. Bradley. 1990. Isolation of a cDNA from the virus responsible for enterically transmitted non-A, non-B hepatitis. Science 247:1335-1339. 73. Riddell, C. 1997. Hepatitis-splenomegaly syndrome. In Diseases of Poultry, p. 1041. Edited by Calnek B. W., Barnes H. J., Beard C. W., McDougald L. R., Saif Y. M. Iowa: Iowa State University Press. 74. Ritchie, S. J., and C. Riddell. 1991. Hepatitis-splenomegaly syndrome in commercial egg-laying hens. Can. Vet. J. 32:500-501. 75. Robinson, R. A., W. H. Burgess, S. U. Emerson, R. S. Leibowitz, S. A. Sosnovtseva, S. Tsarev, and R. H. Purcell. 1998. Structural characterization of 18 recombinant hepatitis E virus ORF2 proteins in baculovirus-infected insect cells. Protein Expr. Purif. 12:75-84. 76. Schlauder, G. G., G. J. Dawson, J. C. Erker, P. Y. Kwo, M. F. Knigge, D. L. Smalley, J. E. Rosenblatt, S. M. Desai, and I. K. Mushahwar. 1998. The sequence and phylogenetic analysis of a novel hepatitis E virus isolated from a patient with acute hepatitis reported in the United States. J. Gen. Virol. 79:447-456. 77. Schlauder, G. G., and I. K. Mushahwar. 2001. Genetic heterogeneity of hepatitis E virus. J. Med. Virol. 65:282-292. 78. Sonoda, H., M. Abe, T. Sugimoto, Y. Sato, M. Bando, E. Fukui, H. Mizuo, M. Takahashi, T. Nishizawa, and H. Okamoto. 2004. Prevalence of hepatitis E virus (HEV) infection in wild boars and deer and genetic identification of a genotype 3 HEV from a boar in Japan. J. Clin. Microbiol. 42:5371-5374. 79. Sun, Z. F., F. F. Huang, P. G. Halbur, S. K. Schommer, F. W. Pierson, T. E. Toth, and X. J. Meng. 2003. Use of heteroduplex mobility assays (HMA) for presequencing screening and identification of variant strains of swine and avian hepatitis E viruses. Vet Microbiol. 96:165-176. 80. Sun, Z. F., C. T. Larsen, A. Dunlop, F. F. Huang, F. W. Pierson, T. E. Toth, and X. J. Meng. 2004. Genetic identification of an avian hepatitis E virus (avian HEV) from healthy chicken flocks and characterization of the capsid gene of 14 avian HEV isolates from chickens with hepatitis-splenomegaly syndrome in different geographic regions of the United States. J. Gen. Virol. 85:693-700. 81. Sun, Z. F., C. T. Larsen, F. F. Huang, P. Billam, F. W. Pierson, T. E. Toth, and X. J. Meng. 2004. Generation and infectivity titration of an infectious stock of avian hepatitis E virus (HEV) in chickens and cross-species infection of turkeys with avian HEV. J. Clin. Microbiol. 42:2658-2662. 82. Takahashi, K., J. H. Kang, S. Ohnishi, K. Hino, H. Miyakawa, Y. Miyakawa, H. Maekubo, and S. Mishiro. 2003. Full-length sequences of six hepatitis E virus isolates of genotypes III and IV from patients with sporadic acute or fulminant hepatitis in Japan. Intervirology 46:308-318. 19 83. Takahashi, K., N. Kitajima, N. Abe, and S. Mishiro. 2004. Complete or nearcomplete nucleotide sequences of hepatitis E virus genome recovered from a wild boar, a deer, and four patients who ate the deer. Virology 330:501-505. 84. Takahashi, M., T. Nishizawa, A. Yoshikawa, S. Sato, N. Isoda, K. Ido, K. Sugano, and H. Okamoto. 2002. Identification of two distinct genotypes of hepatitis E virus in a Japanese patient with acute hepatitis who had not travelled abroad. J. Gen. Virol. 83:1931-1940. 85. Takahashi, M., T. Nishizawa, H. Miyajima, Y. Gotanda, T. Iita, F. Tsuda, and H. Okamoto. 2003. Swine hepatitis E virus strains in Japan form four phylogenetic clusters comparable with those of Japanese isolates of human hepatitis E virus. J. Gen. Virol. 84:851-862. 86. Tam, A. W., M. M. Smith, M. E. Guerra, C. C. Huang, D. W. Bradley, K. E. Fry, and G. R. Reyes. 1991. Hepatitis E virus (HEV): molecular cloning and sequencing of the full-length viral genome. Virology 185:120-131. 87. Tanaka, H., H. Yoshino, E. Kobayashi, M. Takahashi, and H. Okamoto. 2004. Molecular investigation of hepatitis E virus infection in domestic and miniature pigs used for medical experiments. Xenotransplantation 11:503-510. 88. Tei, S., N. Kitajima, K. Takahashi, and S. Mishiro. 2003. Zoonotic transmission of hepatitis E virus from deer to human beings. Lancet 362:371-373. 89. Thomas, D. L., P. O. Yarbough, D. Vlahov, S. A. Tsarev, K. E. Nelson, A. J. Saah, and R. H. Purcell. 1997. Seroreactivity to hepatitis E virus in areas where the disease is not endemic. J. Clin. Microbiol. 35:1244-1247. 90. Tien, N. T., H. T. Clayson, H. B. Khiem, P. K. Sac, A. L. Corwin, K. S. Myint, and D. W. Vaughn. 1997. Detection of immunoglobulin G to the heptatitis E virus among several animal species in Vietnam. Am. J. Trop. Med. Hyg. 57:211 (abstract). 91. Tsarev, S. A., S. U. Emerson, G. R. Reyes, T. S. Tsareva, L. J. Legters, I. A. Malik, M. Iqbal, and R. H. Purcell. 1992. Characterization of a prototype strain of hepatitis E virus. Proc. Natl. Acad. Sci. USA. 89:559-563. 92. Tsarev, S. A., T. S. Tsareva, S. U. Emerson, A. Z. Kapikian, J. Ticehurst, W. London, and R. H. Purcell. 1993. ELISA for antibody to hepatitis E virus (HEV) 20 based on complete open-reading frame-2 protein expressed in insect cells: identification of HEV infection in primates. J. Infect. Dis. 168:369-378. 93. Turkoglu, S., Y. Lazizi, H. Meng, A. Kordosi, P. Dubreuil, B. Crescenzo, S. Benjelloun, P. Nordmann, and J. Pillot. 1996. Detection of hepatitis E virus RNA in stools and serum by reverse transcription-PCR. J. Clin. Microbiol. 34:1568-1571. 94. Tyagi, S., H. Korkaya, M. Zafrullah, S. Jameel, and S. K. Lal. 2002. The phosphorylated form of the ORF3 protein of hepatitis E virus interacts with its nonglycosylated form of the major capsid protein, ORF2. J. Biol. Chem. 277:2275922767. 95. van der Poel, W. H, F. Verschoor, R. van der Heide, M. I. Herrera, A. Vivo, M. Kooreman, and A. M. de Roda Husman. 2001. Hepatitis E virus sequences in swine related to sequences in humans, The Netherlands. Emerg. Infect. Dis. 7:970-976. 96. Wang, Y. C., H. Y. Zhang, N. S. Xia, G. Peng, H. Y. Lan, H. Zhuang, Y. H. Zhu, S. W. Li, K. G. Tian, W. J. Gu, J. X. Lin, X. Wu, H. M. Li, and T. J. Harrison. 2002. Prevalence, isolation, and partial sequence analysis of hepatitis E virus from domestic animals in China. J. Med. Virol. 67:516-521. 97. Williams, T. P. E., Kasorndorkbua C., Halbur P. G., Haqshenas G., Guenette D. K., Toth T. E., and Meng X. J. 2001. Evidence of extrahepatic sites of replication of the hepatitis E virus in a swine model. J. Clin. Microbiol. 39:3040-3046. 98. Withers, M. R., M. T. Correa, M. Morrow, M. E. Stebbins, J. Seriwatana, W. D. Webster, M. B. Boak, and D. W. Vaughn. 2002. Antibody levels to hepatitis E virus in North Carolina swine workers, non-swine workers, swine, and murids. Am. J. Trop. Med. Hyg. 66:384-388. 99. World Health Organization. Department of Communicable Disease Surveillance and Response. 2001. Hepatitis E. WHO/CDS/CSR/EDC/2001.12. 1-28. 100. Wu, J. C., C. M. Chen, T. Y. Chiang, W. H. Tsai, W. J. Jeng, I. J. Sheen, C. C. Lin, and X. J. Meng. 2002. Spread of hepatitis E virus among different-aged pigs: two-year survey in Taiwan. J. Med. Virol. 66:488-492. 101. Yazaki, Y., H. Mizuo, M. Takahashi, T. Nishizawa, N. Sasaki, Y. Gotanda, and H. Okamoto. 2003. Sporadic acute or fulminant hepatitis E in Hokkaido, Japan, may 21 be food-borne, as suggested by the presence of hepatitis E virus in pig liver as food. J. Gen. Virol. 84:2351-2357. 102. Yoo, D., P. Willson, Y. Pei, M. A. Hayes, A. Deckert, C. E. Dewey, R. M. Friendship, Y. Yoon, M. Gottschalk, C. Yason, and A. Giulivi. 2001. Prevalence of hepatitis E virus antibodies in Canadian swine herds and identification of a novel variant of swine hepatitis E virus. Clin. Diagn. Lab. Immunol. 8:1213-1219. 103. Zafrullah, M., M. H. Ozdener, S. K. Panda, and S. Jameel. 1997. The ORF3 protein of hepatitis E virus is a phosphopretein that associates with the cytoskeleton. J. Virol. 71:9045-9053. 104. Zanetti, A. R., G. G. Schlauder, L. Romano, E. Tanzi, P. Fabris, G. J. Dawson, and I. K. Mushahwar. 1999. Identification of a novel variant of hepatitis E virus in Italy. J. Med. Virol. 57:356-360. 105. Zhang, M., S. U. Emerson, H. Nguyen, R. Engle, S. Govindarajan, W. C. Blackwelder, J. Gerin, and R. H. Purcell. 2002. Recombinant vaccine against hepatitis E: duration of protective immunity in rhesus macaques. Vaccine 20:32853291. 22 CHAPTER II Use of Heteroduplex Mobility Assays (HMA) for PreSequencing Screening and Identification of Variant Strains of Swine and Avian Hepatitis E Viruses Z. F. Sun, F. F. Huang, P. G. Halbur, S. K. Schommer, F. W. Pierson, T. E. Toth, and X. J. Meng Veterinary Microbiology, 2003 Oct 17;96(2):165-176. Modified and re-printed with permission. 2.1 Abstract Hepatitis E virus (HEV), the causative agent of human hepatitis E, is an important public health problem in many developing countries and is also endemic in many industrialized countries including the United States. The discoveries of swine and avian HEVs by our group from pigs and chickens, respectively, suggest that hepatitis E may be a zoonosis. Current methods for molecular epidemiological studies of HEV require PCR amplification of field strains of HEV followed by DNA sequencing and sequence analyses, which are laborious and expensive. As novel or variant strains of HEV continue to evolve rapidly both in humans and other animals, it is important to develop a rapid presequencing screening method to select field isolates for further molecular characterization. In this study, we developed two heteroduplex mobility assays (HMA) (one for swine HEV based on the ORF2 region, and the other for avian HEV based on the ORF1 region) to genetically differentiate field strains of swine and avian HEVs from known reference strains. The ORF2 regions of 22 swine HEV isolates and the ORF1 regions of 13 avian HEV isolates were amplified by PCR, sequenced and analyzed by HMA against reference prototype swine HEV strain and reference prototype avian HEV strain, respectively. We showed that, in general, the HMA profiles correlate well with nucleotide sequence identities and with phylogenetic clustering between field strains and 23 the reference swine HEV or avian HEV strains. Field isolates with similar HMA patterns generally showed similar sequence identities with the reference strains and clustered together in the phylogenetic trees. Therefore, by using different HEV isolates as references, the HMA developed in this study can be used as a pre-sequencing screening tool to identify variant HEV isolates for further molecular epidemiological studies. 24 2.2 Introduction Hepatitis E is an important public health problem in many developing countries, and is also endemic in many industrialized countries including the United States (Aggarwal and Krawczynski, 2000; Erker et al., 1999; Favorov et al., 2000; Huang et al., 1992; Mast et al., 1997; Meng et al., 2000a, b, 2002; Purcell, 1996; Schlauder et al., 1998; Schlauder and Mushahwar, 2001; Takahashi et al., 2001; Thomas et al., 1997; Wang et al.,1999, 2000). It has been reported that hepatitis E has a high mortality rate (up to 20%) in infected pregnant women (Hussaini et al., 1997; Purcell, 1996). Hepatitis E virus (HEV), the causative agent of human hepatitis E, is currently unclassified (Aggarwal and Krawczynski, 2000; Purcell, 1996; Schlauder and Mushahwar, 2001). The positive sense viral RNA genome is about 7.2 kb, containing three open reading frames (ORF). ORF1 encodes viral nonstructural proteins, ORF2 encodes the capsid protein and ORF3 encodes a small phosphorylated protein (Huang et al., 1992; Purcell, 1996; Takahashi et al., 2001; Tsarev et al., 1992; Wang et al., 1999, 2000; Zafrullah et al., 1997). Swine hepatitis E virus (swine HEV) was first isolated and characterized from a pig in the U.S. in 1997 (Meng et al., 1997). Swine HEV is widely distributed in pigs worldwide (Clayson et al., 1995; Garkavenko et al., 2001; Halbur et al., 2001; Huang et al., 2002b; Meng et al., 1999; Okamoto et al., 2001; van der Poel et al., 2001; Wu et al., 2002; Yoo et al., 2001). Swine HEV is antigenically and genetically related to human HEV (Meng et al., 1998; Haqshenas et al., 2002). It has been shown that field isolates of swine HEV from pigs in different geographic regions are genetically heterogenic (Huang et al., 2002b). More recently, another animal strain of HEV, avian hepatitis E virus (avian HEV), was isolated and characterized from chickens with Hepatitis-Splenomegaly Syndrome (HS syndrome) in the United States (Haqshenas et al., 2001). It has been shown that avian HEV is genetically and antigenically related to human and swine HEVs (Haqshenas et al., 2001; Haqshenas et al., 2002). Like swine and human HEVs, field isolates of the newly discovered avian HEV from different geographic regions are also genetically very heterogenic (Huang et al., 2002a). The heterogenic nature of these novel animal strains of HEV makes it difficult for large-scale molecular epidemiological studies. The current method to genetically differentiate these new animal strains of HEV 25 include RT-PCR amplification of HEV isolates followed by DNA sequencing (Garkavenko et al., 2001; Huang et al., 2002a, b; Okamoto et al., 2001; van der Poel et al., 2001; Yoo et al., 2001), which is expensive and laborious. Many field isolates with either identical sequence or extremely similar sequence were unnecessarily amplified and sequenced. Therefore, a rapid pre-sequencing screening assay is needed to select variant field isolates of HEV for further genetic characterization. Heteroduplex mobility assay (HMA) is a technique for rapid genetic characterization of closely related sequences (Berinstein et al., 2001; Berry et al., 2001; Fack et al., 2000; Mattick et al., 2000; White et al., 2000). The principle of HMA is the formation of nucleotide mismatches when two divergent DNA molecules are mixed, denatured and allowed to reanneal (Fack et al., 2000). Heteroduplexes are formed when two non-identical but closely related single-strand DNA fragments anneal. By using known reference strains of swine HEV or avian HEV, variant field isolates of HEV could be identified prior to sequencing, for further genetic characterization, based on the formation of homoduplexes (field isolates with identical or very similar sequence with the reference strain) or heteroduplexes (variant field isolates of HEV genetically divergent from the reference strain). The structural distortions at mismatched or unpaired bases cause the heteroduplexes to migrate with reduced mobility in polyacrylamide gel electrophoresis (PAGE). The reduction in mobility (or gel shift) is proportional to the divergence between the sequence of the reference strain and that of the field isolate (Mattick et al., 2000; White et al., 2000). HMA has been shown to detect nucleotide sequence divergence as low as 1% (White et al., 2000). The objective of this study is to develop HMA assays based upon the available ORF2 region of swine HEV genome and the available ORF1 region of avian HEV genome for rapid pre-sequencing screening variant field strains of avian and swine HEVs for further molecular characterization. 2.3 Materials and Methods 2.3.1 Virus Isolates Twenty-two previously characterized field isolates of swine HEV (Table 2.1) used in this study were recovered from pigs of 2-4 months of age in six U.S. states 26 (Huang et al., 2002b). Thirteen previously characterized field isolates of avian HEV (Table 2.1) were identified from chickens with HS syndrome in different geographic regions of the United States (Huang et al., 2002a). Prototype strain of swine HEV (Meng et al., 1997) and prototype strain of avian HEV (Haqshenas et al., 2001) were used as reference strains for the swine HEV HMA and avian HEV HMA, respectively. 2.3.2 RT-PCR Amplification of Different Swine and Avian HEV Isolates Primers used for amplification of swine HEV ORF2 region and avian HEV ORF1 region and the PCR parameters were described previously (Huang et al., 2002a, b). For swine HEV, a nested set of degenerate primers were used to amplify the ORF2 region, since the ORF2 genes for the majority of swine HEV isolates are known: external primer set 3156N [forward, 5'-AATTATGCC(T)CAGTAC(T)CGG(A)GTTG-3'] and 3157N [reverse, 5'-CCCTTA(G)TCC(T) TGCTGA(C)GCATTCTC-3'], and internal primer set 3158N [forward, 5'-GTT(A)ATGCTT(C)TGCATA(T)CATGGCT-3'] and 3159N [reverse, 5'-AGCCGACGAAATCAATTCTGTC-3']. The expected PCR product for swine HEV was 347 bp. For avian HEV, the ORF1 region of most avian HEV isolates is sequenced, and therefore two primers were selected from the helicase gene region in the ORF1 based on a multiple sequence alignment of avian HEV and other strains of HEV (Huang et al., 2002a): B-1 [forward, 5'-GCTAGGCGACCCGCACCAGAT-3'] and B-2 [reverse, 5'GGTTAGCGCAACAATAGCATG-3']. The expected PCR product for avian HEV was 374 bp (Huang et al., 2002a). Twenty-two swine HEV isolates and thirteen avian HEV isolates were amplified by RT- PCR as previously described (Huang et al., 2002a, b) (Table 2.1). 2.3.3 HMA of Swine and Avian HEV Isolates PCR products amplified from each of the 22 swine HEV isolates were mixed separately with the PCR product from the reference prototype strain of swine HEV. Similarly, PCR products from each of the 13 avian HEV isolates were mixed with that of 27 the reference prototype strain of avian HEV. The mixed samples were denatured at 94ºC for 5 min, and allowed to reanneal at 50ºC for 30 min (Mattick et al., 2000; White et al., 2000). Heteroduplexes and homoduplexes were separated by 8% PAGE at 200 V for 5 h. Gels were stained with ethidium bromide and visualized under UV light. 2.3.4 Correlation of HMA Profiles with Sequence Analyses of Amplified Genomic Region(s) of Swine and Avian HEV Isolates PCR products amplified from 22 swine HEV isolates and 13 avian HEV isolates were directly sequenced at the Virginia Tech DNA Sequence Facility and the sequences have been reported previously (Huang et al., 2002a, b). The resulting sequences of the field strains of avian and swine HEVs were analyzed and the percentages of nucleotide sequence identities with respective reference strains were determined (Table 2.1). Isolates with different heteroduplexes were analyzed by sequence comparisons to determine if the heteroduplexes correlated with the percentages of sequence divergence (Table 2.1). 2.3.5 Correlation of HMA Results with Phylogenetic Analyses of the Sequences of Swine and Avian HEV Isolates The PCR primer sequences were excluded from the resulting sequences. The 304 bp sequences in the ORF2 genes of the 22 U.S. isolates of swine HEV and the 332 bp sequences in the ORF1 genes of the 13 U.S. isolates of avian HEV were phylogenetically analyzed along with the respective reference avian HEV or swine HEV strains. Phylogenetic analyses were conducted with the aid of the PAUP program (David L. Swofford, Smithsonian Institute, Washington DC). Heuristic search with 1,000 replicates was used to produce a phylogenetic tree. The tree topologies were compared to the heteroduplex patterns in the gels to determine if the heteroduplex patterns correlated with the phylogenetic clustering of the swine or avian HEV isolates. 28 2.3.6 GenBank Accession Numbers The GenBank accession numbers for the nucleotide sequences of the 22 swine HEV isolates as well as the prototype swine HEV used in the study are: 10017 (AY870833), 14185.1 (AF466685), 15555A (AF466683), 16137.1 (AF466668), 16137.2 (AY870834), 16138 (AY871094), 16138.2 (AF466669), 16138.3 (AF466670), 16139.2 (AF466671), 18356.1 (AF466663), 18934C1 (AF466662), 18934D1 (AF466661), 19248.3 (AF466660), 21160.1 (AF66659), 22171B1 (AF466673), 22807.2 (AF466680), 31692C1 (AF466682), 9913 (AF466676), UMC7A (AF466667), UMC7B (AF466664), UMC9A (AF466666), UMC9B (AY870835), and prototype swine HEV (AF082843). The GenBank accession numbers for the nucleotide sequences of the 13 avian HEV isolates as well as the prototype avian HEV used in the study are: CA077 (AF531908), CA240 (AY871091), CA242 (AF531898), CA518.5 (AY870832), CA697A (AF531907), CA697B (AF531906), CT090A (AF531905), CT090A.2 (AY871092), CT690.2 (AF531899), CT690.2.2 (AY871093), NY449A (AF531902), WI318B (AF531901), WI966G (AF531900) and prototype avian HEV (AY043166). The GenBank accession number for the nucleotide sequence of HEV Sar-55 strain used in the phylogenetic analysis is M80581. 2.4 Results 2.4.1 HMA of Swine and Avian HEV Isolates with Respective Reference Viruses PCR products of 22 swine HEV isolates and 13 avian HEV isolates were mixed with the PCR products from the reference prototype swine HEV strain and the reference prototype avian HEV strain, respectively, and subjected to HMA. HMA analyses showed that different swine HEV isolates and different avian HEV isolates have different mobilities in HMA gels. In general, there is a greater mobility reduction of heteroduplexes for avian HEV isolates (Fig. 2.1C) compared to the mobility reduction of swine HEV isolates (Figs. 2.1A and 2.1B). This is due to the increased sequence divergence among the avian HEV isolates varying from 14 to 23% (Table 2.1), which is 29 much higher than the sequence variation among swine HEV isolates (4 to 11%) (Table 2.1). 2.4.2 Correlation of HMA Results with Percentage of Sequence Identities of Amplified Genomic Region(s) of Swine and Avian HEV Isolates Swine HEV isolates with similar HMA patterns (Figs. 2.1A and 2.1B) generally had similar sequence identity with the reference prototype swine HEV strain (Table 2.1). As expected, the PCR product of the prototype swine HEV formed a homoduplex with the reference swine HEV strain as their sequences are identical (Figs. 2.1A and 2.1B). Swine HEV field isolate 9913, which shared 96% nucleotide sequence identity with the reference swine HEV strain, formed a HMA pattern similar to a homoduplex (Fig. 2.1A). All other field isolates of swine HEV, which shared only 89-93% nucleotide sequence identities with the reference strain, formed heteroduplexes with varying degree of mobility reduction (Figs. 2.1A and 2.1B). For examples, UMC9A and UMC9B only have 89% sequence identities with the prototype swine HEV, and thus they have the highest mobility reduction in the HMA. UMC7B, 21160.1 and 19248.3 isolates have 91-92% sequence identities with the prototype swine HEV, and thus they have less mobility reduction than UMC9A and UMC9B isolates. Similar to swine HEV, the avian HEV HMA results revealed that avian HEV isolates with similar HMA patterns (Fig. 2.1C) generally had similar sequence identity with the reference avian HEV strain (Table 2.1). Homoduplex is formed when the identical prototype avian HEV isolate was used in the HMA. Field isolates WI318B and WI966G, which shared 85-86% sequence identities with the reference strain, and formed similar heteroduplexes (Fig. 2.1C). All other field isolates, sharing 77-81% sequence identities with reference strain, formed heteroduplexes that are not readily distinguishable (Fig. 2.1C). Field isolates of avian HEV identified from the same flock (CA240 and CA242, CT690.2 and CT690.2.2, or CT090A and CT090A.2) had very similar HMA patterns (Fig. 2.1C). 30 2.4.3 Correlation of HMA Results with Phylogenetic Analyses of Swine and Avian HEV Sequences of Targeted Genomic Region(s) Phylogenetic analyses of swine HEV showed that, in general, swine HEV isolates with similar HMA patterns clustered together (Fig. 2.2A). For example, isolates UMC7A and UMC9A had similar HMA patterns (Fig. 2.1B) and percentage of sequence identities with the reference strain (Table 2.1) and they clustered together in a minor branch (Fig. 2.2A). Isolates 16137.1, 16138.2, 16138.3 and 16139.2 had similar HMA patterns (Fig. 2.1A) and are also clustered together (Fig. 2.2A). Field isolate 9913 of swine HEV had a 96% nucleotide sequence identity with the reference prototype swine HEV strain and formed a homoduplex HMA with the reference prototype swine HEV strain (Fig. 2.1A). As expected, isolate 9913 clustered in the same branch with the reference prototype swine HEV (Fig. 2.2A). Similar to swine HEV, phylogenetic analyses of avian HEV isolates also showed that isolates with similar HMA patterns clustered together (Fig. 2.2B). For examples, the two field isolates of avian HEV (WI318B and WI966G) with similar HMA patterns and similar genetic similarity with the reference strain (85-86% identity) (Table 2.1) are phylogenetically closely-related (Fig. 2.2B). Isolates CA697A and CA697B were identified from the same flock and had similar HMA patterns and similar sequence identities (77-78%) with the reference strain, and thus these two isolates also clustered together phylogenetically. 2.5 Discussion HEV is an important human pathogen. The recent discoveries of geneticallyrelated novel strains of HEV from swine (swine HEV) and chickens (avian HEV) suggest that HEV may be zoonotic (Haqshenas et al., 2001; Kabrane-Lazizi et al., 1999; Meng et al., 1997; Meng, 2000a, b; Tien et al., 1997). As variant strains of HEV continue to evolve in human and other animal species, there is a need for a rapid assay to distinguish novel variant strains from those known strains and to pre-screen field isolates for further genetic characterization. Here in this study, we developed two HMA assays for rapid genetic characterization of avian HEV and swine HEV isolates, respectively. We found 31 that, in general, swine HEV isolates identified from the same herds or same geographic regions displayed similar HMA patterns compared to those from different herds or different geographic regions. For example, swine HEV isolates UMC7A, UMC9A and UMC9B from the same geographic region had similar HMA patterns, and shared similar sequence identity with the prototype swine HEV strain and clustered together in the phylogenetic tree. Similarly, swine HEV isolates 16137.1, 16138.2, 16138.3, 16139.2 from the same geographic region had similar HMA patterns. Like swine HEV, avian HEV isolates CA240 and CA242 from the same flock had similar HMA profiles and clustered together in the phylogenetic tree. Overall, we showed that the heteroduplex patterns obtained by HMA generally correlated with sequencing and phylogenetic results. Homoduplexes were formed when two identical viruses were mixed. A homoduplex was formed when a swine HEV field isolate (9913) with 96% sequence identity with the reference strain was assayed. Field isolates with greater sequence divergence with the reference strain formed heteroduplexes with reduced mobility. Therefore, with known strains of HEV as standard references, field isolates of avian and swine HEV can be rapidly characterized to select variant strains for further genetic characterization. The HMA assays have advantages for largescale monitoring and screening programs such as pre-sequencing screening of field isolates and thus reducing the need and cost of having to sequence all the field isolates (Fack et al., 2000). It is known that not only the percentage of sequence identity may affect the HMA patterns but the relative positions of nucleotide mismatches may also play a role (Berinstein et al., 2001; Upchurch et al., 2000). Lack of base pairing at interior positions of two sequences are more likely to affect the HMA pattern than those bases not forming exact pairs at exterior positions. Therefore, PCR products with significant sequence identity may either form homoduplexes if those nonpaired bases are at the exterior positions or form heteroduplexes if at interior positions (Berinstein et al., 2001). Although the PCR products of the swine HEV isolates 14185.1 and 31692C1 shared 92% sequence identity with each other and shared 90% and 91% sequence identity with the reference prototype swine HEV strain, respectively, and they clustered together in the phylogenetic tree, but their HMA patterns were different. This could be due to nucleotide 32 substitutions occurring at interior positions. However, after aligning the sequences of these isolates, we found that there are too many mutations and they appear to be randomly distributed. We also noticed that swine HEV isolate 14185.1 had similar HMA patterns with isolates 21160.1 and 18934D1, and they shared 90%, 92% and 90% sequence identities with the reference strain, respectively, but they clustered in two different minor branches. We also aligned the sequences of these isolates, again the mutations appear to be randomly distributed. Therefore, the observed discrepency between the HMA patterns and phylogenetic trees in the few isolates could not be explained by the locations of the mismatches. In conclusion, we showed that, by using known HEV strains as standard references, HMA is a rapid method for preliminary screening of large numbers of field isolates of HEV for further genetic characterization, and for rapid identification of variant strains of HEV from those closely-related to the known reference strains. 2.6 Acknowledgements We thank Dr. H. L. Shivaprasad and Dr. P. R. Woolcock of the California Animal Health and Food Safety Laboratory System, Fresno, CA for providing the chicken samples. This study is supported in part by grants from the National Institute of Health (AI 01653, AI 46505 and AI 50611) and by a grant from the US Department of Agriculture National Research Initiative Competitive Grants Program (NRI 2002-35204-12531). 33 2.7 References 1. Aggarwal R., Krawczynski K., 2000. Hepatitis E: An overview and recent advances in clinical and laboratory research. J. Gastroenterol. Hepatol. 15, 9-20. 2. Berinstein A., Sellers H. S., King D. J., Seal B. S., 2001. Use of a heteroduplex mobility assay to detect differences in the fusion protein cleavage site coding sequences among Newcastle disease virus isolates. J. Clin. Microbiol. 39, 3171-3178. 3. Berry S., Rey M. E. C., 2001. Differentiation of cassava-infecting begomoviruses using heteroduplex mobility assays. J. Med. Virol. 92, 151-163. 4. Clayson E. T., Innis K. S., Myint S., Narupiti S., Vaughn D. W., Giri S., Ranabhat P., Shrestha M. P., 1995. Detection of hepatitis E virus infections among domestic swine in the Kathmandu Valley of Nepal. Am. J. Trop. Med. Hyg. 53, 228-232. 5. Erker J. C., Desai S. M., Schlauder G.G., Dawson G. J., Mushahwar I. K., 1999. A hepatitis E virus variant from the United States: molecular characterization and transmission in cynomolgus macaques. J. Gen. Virol. 80, 681-690. 6. Fack F., Deroo S., Kreis S., Muller P., 2000. Heteroduplex mobility assay (HMA) pre-screening: An improved strategy for the rapid identification of inserts selected from phage-displayed peptide libraries. Mol. Diversity. 5, 7-12. 7. Favorov M. O., Kosoy M. Y., Tsarev S. A., Childs J. E., Margolis H. S., 2000. Prevalence of antibody to hepatitis E virus among rodents in the United States. J. Infect. Dis. 184, 449-455. 8. Garkavenko O., Obriadina A., Meng J., Anderson D. A., Benard H. J., Schroeder B. A., Khudaakov Y. E., Fields H. A., Croxson M . C., 2001. Detection and characterization of swine hepatitis E virus in New Zealand. J. Med. Virol. 65, 525-529. 9. Halbur P. G., Kasorndorkbua C., Gilbert C., Guenette D., Potters M. B., Purcell R. H., Emerson S. U., Toth T. E., Meng X. J., 2001. Comparative pathogenesis of infection of pigs with hepatitis E viruses recovered from a pig and a human. J. Clin. Microbiol. 39, 918-923. 10. Haqshenas G., Shivaprasad H. L., Woolcock P. R., Read D. H., Meng X. J., 2001. Genetic identification and characterization of a novel virus related to human hepatitis E virus from chickens with hepatitis-splenomegaly syndrome in the United States. J. Gen. Virol. 82, 2449-2462. 34 11. Haqshenas G., Huang F. F., Fenaux M., Guenette D. K., Pierson F. W., Larsen C. T., Shivaprasad H. L., Toth T. E., Meng X. J., 2002. The putative capsid protein of the newly identified avian hepatitis E virus shares antigenic epitopes with that of swine and human hepatitis E viruses and chicken big liver and spleen disease virus. J. Gen. Virol. 83, 2201-2209. 12. Hsieh S. Y., Meng X. J., Wu Y. H., Liu S. T., Tam A. W., Lin D. Y., Liaw Y. F., 1999. Identity of a novel swine hepatitis E virus in Taiwan forming a monophyletic group with Taiwan isolates of human hepatitis E virus. J. Clin. Microbiol. 37, 38283834. 13. Huang C. C., Nguyen D., Fernandez J., Yun K. Y., Fry K. E., Bradley D. W., Tam A. W., Reyes G. R., 1992. Molecular cloning and sequencing of the Mexico isolate of hepatitis E virus (HEV). Virology. 191, 550-558. 14. Huang F. F., Haqshenas G., Shivaprasad H. L., Guenette D. K., Woolcock P. R., Larsen C. T., Pierson F. W., Elvinger F., Toth T. E., Meng X. J., 2002a. Heterogeneity and seroprevalence of the newly identified avian hepatitis E virus from chickens in the United States. J. Clin. Microbiol. 40, 4197-4202. 15. Huang F. F., Haqshenas G., Guenette D. K., Halbur P. G., Schommer S., Pierson F. W., Toth T. E., Meng X. J., 2002b. Detection by RT-PCR and genetic characterization of field isoltes of swine hepatitis E virus from pigs in different geographic regions of the United States. J. Clin. Microbiol. 40, 1326-1332. 16. Hussaini S. H., Skidmore S. J., Richardson P., Sherratt L. M., Cooper B. T., O’Grady J. G., 1997. Severe hepatitis E infection during pregnancy. J. Viral Hepatitis. 4, 51-54. 17. Kabrane-Lazizi Y., Fine J. B., Elm J., Glass G. E., Higa H., Diwan A., Gibbs Jr. C. J., Meng X. J., Emerson S. U., Purcell R. H., 1999. Evidence for wide-spread infection of wild rats with hepatitis E virus in the United States. Am. J. Trop. Med. Hyg. 61, 331- 335. 18. Mast E. E., Kuramoto I. K., Favorov M. O., Schoening V. R., Burkholder B. T., Shapiro C. N., Holland P. V., 1997. Prevalence of and risk factors for antibody to hepatitis E virus seroreactivity among blood donors in Northern California. J. Infect. Dis. 176, 34-40. 35 19. Mattick K. L., Green J., Punia P., Belda F. J., Gallimore C. I., Brown D. W. G., 2000. The heteroduplex mobility assay (HMA) as a pre-sequencing screen for Norwalk-like viruses. J. Virol. Method. 87, 161-169. 20. Meng X. J., 2000a. Zoonotic and xenozoonotic risks of hepatitis E virus. Infect. Dis. Rev. 2, 35-41. 21. Meng X. J., 2000b. Novel strains of hepatitis E virus identified from humans and other animal species: is hepatitis E a zoonosis? J. Hepatol. 33, 842-845. 22. Meng X. J., Dea S., Engle R. E., Friendship R., Lyoo Y. S., Sirinnarumitr T., Urairong K., Wang D., Wong D., Yoo D., Zhang Y., Purcell R. H., Emerson S. U., 1999. Prevalence of antibodies to the hepatitis E virus in pigs from countries where hepatitis E is common or is rare in the human population. J. Med. Virol. 58, 297-302. 23. Meng X. J., Halbur P. G., Shapiro M. S., Govindarajan S., Bruna J. D., Mushahwar I. K., Purcell R. H., Emerson S. U., 1998. Genetic and experimental evidence for cross-species infection by the swine hepatitis E virus. J. Virol. 72, 97149721. 24. Meng X. J., Purcell R. H., Halbur P. G., Lehman J. R., Webb D. M., Tsareva T. S., Haynes J. S., Thacker B. J., Emerson S. U., 1997. A novel virus in swine is closely related to the human hepatitis E virus. Proc. Natl. Acad. Sci. USA. 94, 98609865. 25. Meng X. J., Wiseman B., Elvinger F., Guenette D. K., Toth T. E., Engle R. E., Emerson S. U., Purcell R. H., 2002. Prevalence of antibodies to the hepatitis E virus in veterinarians working with swine and in normal blood donors in the United States and other countries. J. Clin. Microbiol. 40, 117-122. 26. Okamoto H., Takahashi M., Nishizawa T., Fukai K., Muramatsu U., Yoshikawa A., 2001. Analysis of the complete genome of indigenous swine hepatitis E virus isolated in Japan. Biochem. Biophys. Res. Commun. 289, 929-936. 27. Purcell R. H., 1996. Hepatitis E virus. In: Fields B. N., Knipe D. M. and Howley P. M. (Eds.), Field Virology, 3rd edition, Vol. 2, Lippincott-Raven, Philadelphia, pp. 2831-2843. 28. Riddell C., 1997. Hepatitis-splenomegaly syndrome. In: Calnek B. W., Barnes H. J., Beard C. W., McDongald L. R. and Saif Y. M. (Eds.), Diseases of Poultry. Iowa State 36 University Press, Ames, pp. 1041. 29. Schlauder G. G., Dawson G.J., Erker J.C., Kwo P.Y., Knigge M. F., Smalley D. L., Rosenblatt J. E., Desai S. M., Mushahwar I. K., 1998. The sequence and phylogenetic analysis of a novel hepatitis E virus isolated from a patient with acute hepatitis reported in the United States. J. Gen. Virol. 79, 447-456. 30. Schlauder G. G., Mushahwar I. K., 2001. Genetic heterogeneity of hepatitis E virus. J. Med. Virol. 65, 282-292. 31. Takahashi K., Iwata K., Watanabe N., Hatahara T., Ohta Y., Baba K., Mishiro S., 2001. Full-genome nucleotide sequence of a hepatitis E virus strain that may be indigenous to Japan. Virology. 287, 9-12. 32. Thomas D. L., Yarbough P. O., Vlahov D., Tsarev S. A., Nelson K. E., Saah A. J., Purcell R. H., 1997. Seroreactivity to hepatitis E virus in areas where the disease is not endemic. J. Clin. Microbiol. 35, 1244-1247. 33. Tien N. T., Clayson H. T., Khiem H. B., Sac P. K., Corwin A. L., Myint K. S., Vaughn D. W., 1997. Detection of immunoglobulin G to the heptatitis E virus among several animal species in Vietnam. Am. J. Trop. Med. Hyg. 57, 211 (abstract). 34. Tsarev S. A., Emerson S. U., Reyes G. R., Tsareva T. S., Legters L. J., Malik I. A., Iqbal M., Purcell R. H., 1992. Characterization of a prototype strain of hepatitis E virus. Proc. Natl. Acad. Sci. USA. 89, 559-563. 35. Upchurch D. A., Shankarappa R., Mullins J. I., 2000. Position and degree of mismatches and the mobility of DNA heteroduplexes. Nucleic Acids Res. 28, e69. 36. van der Poel W. H, Verschoor F., van der Heide R., Herrera M. I., Vivo A., Kooreman M., de Roda Husman A. M., 2001. Hepatitis E virus sequences in swine related to sequences in humans, the Netherlands. Emerg. Infect. Dis. 7, 970-976. 37. Wang Y., Ling R., Erker J. C., Zhang H., Li H., Desai S., Mushahwar I. K., Harrison T. J., 1999. A divergent genotype of hepatitis E virus in Chinese patients with acute hepatitis. J. Gen. Virol. 80, 169-177. 38. Wang Y., Zhang H., Ling R., Li H., Harrison T. J., 2000. The complete sequence of hepatitis E virus genotype 4 reveals an alternative strategy for translation of open reading frames 2 and 3. J. Gen. Virol. 81, 1675-1686. 37 39. White P. A., Li Z., Zhai X., Marinos G., Rawlinson W. D., 2000. Mixed viral infection identified using heteroduplex mobility analysis (HMA). Virology. 271, 382389. 40. Wu J. C., Chen C. M., Chiang T. Y., Tsai W. H., Jeng W. J., Sheen I. J., Lin C. C., Meng X. J., 2002. Spread of hepatitis E virus among different-aged pigs: two-year survey in Taiwan. J. Med. Virol. 66, 488-492. 41. Yoo D., Willson P., Pei Y., Hayes M. A., Deckert A., Dewey C. E., Friendship R. M., Yoon Y., Gottschalk M., Yason C., Giulivi A., 2001. Prevalence of hepatitis E virus antibodies in Canadian swine herds and identification of a novel variant of swine hepatitis E virus. Clin. Diagn. Lab. Immunol. 8, 1213-1219. 42. Zafrullah M., Ozdener M. H., Panda S. K., Jameel S., 1997. The ORF3 protein of hepatitis E virus is a phosphopretein that associates with the cytoskeleton. J. Virol. 71, 9045-9053. 38 650 kb C prototype avian HEV as the reference (C). 39 CA697B CA697A CA518.5 NY449 WI318B CT090A.2 CT090A CT690.2.2 CT690.2 CA077 WI966G CA242 CA240 Prototype swine HEV 1Kb DNA Marker 1Kb DNA Marker 10017 22807.2 15555A 14185.1 22171B1 16139.2 16138.3 16138.2 16138 16137.1 1Kb DNA Marker Prototype swine HEV Prototype swine HEV 650 kb 9913 1000 kb 850 kb 31692C1 18356.1 UMC9B UMC9A UMC7B UMC7A 21160.1 19248.3 18934D1 18934C1 Prototype swine HEV 850 kb Prototype avian HEV 1000 kb 1Kb DNA Marker B 1Kb DNA Marker A 1650 kb 1000 kb 850 kb 650 kb Fig. 2.1. HMA analyses of swine HEV isolates (A and B) using the prototype swine HEV as the reference, and HMA analyses of avian HEV isolates using Fig. 2.2. A 5 changes 22807.2 14185.1 31692C1 10017 15555A 16137.2 16138 22171B1 16139.2 16138.3 16138.2 16137.1 18934C1 UMC7B 40 Swine HEV USA 9913 18356.1 UMC7A UMC9B UMC9A 18934D1 21160.1 19248.3 HEV Sar-55 B 5 changes NY449A WI966G CT690.2.2 CT690.2 CA518.5 CA242 CA240 CT090A.2 CT090A CA697B CA697A CA077 WI318B Avian HEV USA Fig. 2.2. A. A phylogenetic tree based on the nucleotide sequences of a 304-bp region within the ORF2 gene of swine HEV isolates. B. A phylogenetic tree based on the 332-bp sequences within the helicase gene region of avian HEV isolates used in this study. The tree was constructed with the aid of the PAUP program. A heuristic search with 1,000 replicates and with a midpoint rooting option was used to construct the tree. A scale bar representing the numbers of character state changes is proportional to the genetic distance. The reference prototype swine HEV and avian HEV are shown in bold. 41 Table 2.1. Field isolates of swine and avian hepatitis E viruses used in the HMA analyses and their nucleotide sequence identities with respective reference strains ID numbers Geographic Origin Percentage of nucleotide sequence identities with respective reference strain (%) Swine HEV Prototype swine HEV 9913 18356.1 21160.1 22171B1 UMC7B 10017 31692C1 19248.3 15555A 16137.1 16137.2 16138.2 16138.3 16139.2 16138 18934D1 22807.2 14185.1 18934C1 UMC7A UMC9A UMC9B Holdenville, Okla. Manson, Iowa Cherokee, Iowa Everly, Iowa Columbia, Mo. Kamrar, Iowa Kamrar, Iowa Alden, Iowa Ute, Iowa Bloomfield, Iowa Bloomfield, Iowa Bloomfield, Iowa Bloomfield, Iowa Bloomfield, Iowa Bloomfield, Iowa Linn Grove, Iowa Dayton, Iowa Remsen, Iowa Linn Grove, Iowa Columbia, Mo. Columbia, Mo. Columbia, Mo. 100 96 93 92 92 92 91 91 91 91 91 91 91 91 91 90 90 90 90 89 89 89 89 Avian HEV Prototype avian HEV WI318B WI966G CA518.5 CA240 CA242 NY449 CT690.2 CT690.2.2 CT090A CT090A.2 CA077 CA697B CA697A WI WI CA CA CA NY CT CT CT CT CA CA CA 100 86 85 81 81 81 80 79 79 79 79 78 78 77 42 CHAPTER III Genetic Identification of An Avian Hepatitis E Virus (HEV) from Healthy Chicken Flocks and Characterization of the Capsid Gene of 14 Avian HEV Isolates from Chickens with Hepatitis-Splenomegaly Syndrome in Different Geographic Regions of the United States Z. F. Sun, C. T. Larsen, A. Dunlop, F. F. Huang, F. W. Pierson, T. E. Toth, and X. J. Meng Journal of General Virology, 2004 Mar;85(Pt 3):693-700. Modified and re-printed with permission. 3.1 Abstract Avian hepatitis E virus (avian HEV), a novel virus identified from chickens with hepatitis-splenomegaly (HS) syndrome, is genetically and antigenically related to human HEV. Recently, we found that avian HEV antibody is also prevalent in healthy chickens. A prospective study was conducted on a known seropositive but healthy chicken farm to identify avian HEV isolates from healthy chickens. Fourteen chickens were randomly selected from this farm, tagged and monitored under natural conditions for 19 weeks. All 14 chickens were seronegative at the beginning of the study at 12 weeks of age. By 21 weeks of age, all 14 chickens had seroconverted to avian HEV antibody. None of the chickens had any sign of HS syndrome. Partial helicase gene and capsid gene sequences of avian HEV isolates recovered from four healthy chickens were determined, and found to share 70-97% nucleotide sequence identities with the corresponding regions of avian HEV isolates from chickens with HS syndrome. Recovery of identical viruses from the experimentally inoculated chickens in the subsequent transmission study further confirmed our field results. Thus far only one strain of avian HEV from a chicken with HS syndrome has been genetically characterized for its capsid gene. To determine the extent of genetic variation among avian HEV isolates, we characterized the capsid gene 43 region of an additional 14 isolates from chickens with HS syndrome. We found that the capsid genes of avian HEV isolates from chickens with HS syndrome are heterogeneic, sharing 76-100% nucleotide sequence identities to each other. The study indicates that avian HEV is enzootic in chicken flocks and spread subclinically among chickens, and that the virus is heterogeneic. 44 3.2 Introduction Hepatitis E virus (HEV), the causative agent of human hepatitis E, is an important human pathogen (Purcell, 1996; Reyes, 1997; Aggarwal and Krawezynski, 2000). HEV is a positive-sense, single-strand, non-enveloped RNA virus. The genome of HEV is about 7.2 kb and contains three open reading frames (ORFs) (Huang et al., 1992; Tsarev et al., 1992; Purcell, 1996; Reyes, 1997; Schlander et al., 1998; Emerson et al., 2001). Hepatitis E is primarily transmitted through the fecal-oral route with an incubation period of about 15-60 days. Although the mortality rate is generally low (less than 1%) in young adults, it can be up to 15-25% in infected pregnant women (Purcell, 1996; Hussaini et al., 1997; Reyes, 1997). Hepatitis E is an important public health concern in many developing countries (Huang et al., 1992; Arankalle et al., 1994; Purcell, 1996; Wang et al., 1999, 2000, 2002). Although only sporadic cases of acute hepatitis E have been reported in industrialized countries including the United States (Schlauder et al., 1998, 1999; Erker et al., 1999; Zanetti et al., 1999; Pina et al., 2000; McCrudden et al., 2000; Takahashi et al., 2001, 2002, 2003b; Clemente-Casares et al., 2003), a significant proportion of healthy individuals in industralized countries are seropositive for HEV antibodies (Hsieh et al., 1999; Mast et al., 1997; Thomas et al., 1997; Meng et al., 1999, 2002; Meng, 2000a, b, 2003). Increasing evidence indicated that hepatitis E is a zoonosis (Meng et al., 1997, 1998, 1999, 2002; Kabrane-Lazizi et al., 1999; Favorov et al., 2000; Meng, 2000a, b, 2003; Nishizawa et al., 2003; Takahashi et al., 2003a, b; Tei et al., 2003). Swine HEV, the first animal strain of HEV, was first discovered and characterized from a pig in the United States in 1997 (Meng et al., 1997) and since then many swine HEV isolates have been identified worldwide and shown to be genetically close-related to strains of human HEVs (Chandler et al., 1999; Hsieh et al., 1999; Pina et al., 2000; Halbur et al., 2001; Haqshenas & Meng, 2001a; Garkavenko et al., 2001; Okamoto et al., 2001; van der Poel et al., 2001; Williams et al., 2001; Huang et al., 2002b; Kasorndorkbua et al., 2002; Wu et al., 2002; Takahashi et al., 2003a, b). Recently, another animal strain of HEV, avian HEV, was identified in the United States from chickens with hepatitis-splenomegaly (HS) syndrome (Haqshenas et al., 2001b), and showed to be antigenically and genetically related to human and swine HEVs (Haqshenas 45 et al., 2001b; 2002; Huang et al., 2002a). HS syndrome was first reported in 1991 in western Canada and has now been recognized in the United States (Ritchie & Riddell, 1991). The disease is characterized by increased mortality in broiler breeder and laying hens of 30-72 weeks of age with up to 20% drop in egg production (Ritchie & Riddell, 1991). Regressive ovaries, red fluid in the abdomen, enlarged liver and spleen were usually seen in infected chickens with histological changes of hepatic necrosis and hemorrhage (Ritchie & Riddell, 1991; Shivaprasad & Woolcock, 1995; Riddell, 1997). It has been shown that avian HEV shares approximately 50-60% nucleotide sequence identities with the known human and swine HEVs and about 80% sequence identity with the Australian chicken big liver and spleen disease virus (BLSV) (Payne et al., 1999; Haqshenas et al., 2001b). Our recent study showed that avian HEV antibody is highly prevalent in apparently healthy chicken flocks in the United States (Huang et al., 2002a). However, thus far avian HEV has only been genetically identified from chickens with HS syndrome (Haqshenas et al., 2001b; Huang et al., 2002a; Sun et al., 2003). Therefore, it is important to genetically identify and characterize avian HEV from chickens without clinical disease. In addition, the extent of genetic varibility of the ORF2 capsid gene of avian HEV isolates from chickens with HS syndrome is not known, as only one strain of avian HEV has been sequenced so far for the ORF2 capsid gene. Thus, it is also important to characterize the capsid gene sequences of avian HEV isolates from chickens with HS syndrome in different geographic regions. 3.3 Materials and Methods 3.3.1 Clinical Samples Bile samples used for the genetic charaterization of the ORF2 capsid gene of avian HEV isolates were collected from 14 chickens with HS syndrome in California, Conneticut, New York, and Wisconsin (Huang et al., 2002a). Clinical samples (serum and fecal materials) used for genetic identification and characterization of an avian HEV isolate from healthy chickens are collected from the prospective study described below. 46 3.3.2 Prospective Study We have previously shown that avian HEV antibodies are highly prevalent not only in chicken flocks with HS syndrome but in healthy chicken flocks with no history of diseases as well (Huang et al., 2002a). In order to genetically identify the virus responsible for the seropositivity in healthy chickens, we conducted a prospective study. Briefly, fourteen 12-week-old chickens were randomly selected from 3 healthy chicken flocks in a commercial farm in Virginia that had previously tested positive for avian HEV antibody (Huang et al., 2002a). Each of the 14 chickens was tagged and mixed with other chickens in the same flock. The 14 study chickens were housed and raised under the same natural conditions as the other chickens in the flocks. Weekly or biweekly fecal swabs and serum samples were collected from the 14 chickens for 19 weeks, and the chickens were 30 weeks of age at the end of the prospective study. Both the serum and fecal samples were tested by RT-PCR for avian HEV RNA, and the serum samples were also tested by an ELISA for avian HEV antibody. 3.3.3 Primer Design Primers used for genetic identification and characterization of avian HEV isolates were designed from the helicase gene region in ORF1 as well as from ORF2 gene region based on multiple sequence alignments of prototype avian HEV and other HEV isolates (Haqshenas et al., 2001b; Huang et al., 2002a; Sun et al., 2003). For amplication of the helicase gene region, two nested sets of degenerate primers were used. The primer sequences are: external primer set, AHEV F-1/SD: 5’TGTTATT(C)ACACCCACCAAG(A)ACGT(C)TG-3’; Helic R: 5’CCTCA(G)TGGACCGTA(T)ATCGACCC-3’; internal primer set, AHEV F-2/SD: 5’GCCACGGCTG(A)TTACACCC(T)CAC(T)GT-3’; Helic R-2: 5’GACCCA(G)GGA(G)TTCGACTGCTT-3’. The sizes of expected PCR products for the first and second rounds were 452 bp and 386 bp, respectively. For amplication of the ORF2 capsid gene region, two nested sets of degenerate primers were used. The primer sequences are: external primer set, AHEV ORF2/F-1/SD: 5’-TCGCCT(C)GGTAAT(C)ACA(T)AATGC-3’; AHEV ORF2/R-1/SD: 5’- 47 GCGTTC(G)CCG(C)ACAGGT(C)CGGCC-3’; internal primer set, AHEV ORF2/F2/SD: 5’-ACA(T)AATGCT(C)AGGGTCACCCG-3’; AHEV ORF2/R-2/SD: 5’ATGTACTGA(G)CCA(G)CTG(C)GCCGC-3’. The sizes of expected PCR products for the first and second rounds were 278 bp and 242 bp, respectively. 3.3.4 RT-PCR RNA was extracted with TriReagent (Molecular Research Center, Inc.) from 100 µl of chicken fecal, bile or serum samples. Total RNA was resuspended in 12.25 µl of DNase-free, RNase-free, and proteinase-free water. Reverse transcription was performed at 42°C for 60 min in the presence of a master mix consisting of 12.25 µl of total RNA, 0.25 µl of Superscript II reverse transcriptase (Invitrogen), 1 ul of 10 µM antisense primer, 0.5 µl of RNase inhibitor, 1 µl of 0.1M dithioteritol, 4 µl of 5x RT buffer, and 1 µl of 10 mM dNTPs. The resulting cDNA was amplified by PCR with appropriate primers, and AmpliTaq Gold DNA polymerase (Applied Biosystems). The PCR reaction parameters consisted of an initial incubation at 95°C for 9 min, followed by 39 cycles of amplification at 94°C for 1 min for denaturation, 42°C for 1 min for annealing and 72°C for 1.5 min for extension, followed by a final incubation period at 72°C for 7 min. The PCR products were examined on a 0.8% agarose gel. 3.3.5 ELISA to Detect Anti-Avian HEV Antibody in Chickens A truncated recombinant ORF2 capsid protein of avian HEV was expressed in Escherichia Coli stain BL21StarTM (DE3)pLyS (Invitrogen) and purified by a modified affinity chromatography using the BugBuster His-Bind Purification Kit (Novagen) (Haqshenas et al., 2002). The purified protein was used as the antigen to standardize an enzyme-linked immunosorbent assay (ELISA) to detect avian HEV antibodies in chickens as reported previously (Huang et al., 2002a). Briefly, the purified ORF2 antigen of avian HEV was coated onto 96-well flat bottom microtiter plates (Thermo Labsystems). A horseradish peroxidase (HRP)-conjugated rabbit anti-chicken IgG (Sigma) was used as the secondary antibody. All sera were tested in duplicate at a dilution of 1:100 in 0.05% Tween 20 - phosphate-buffered saline (PBS) blocking buffer 48 containing 5% natural nonfat dry milk fortified with Vitamins A & D (Carnation) and 5% goat serum (Gibco-BRL). Sera from SPF chickens were used as negative controls, and convalescent sera from SPF chickens experimentally infected with avian HEV were included as positive controls. All sera were tested at least twice. 3.3.6 Sequence and Phylogenetic Analyses The PCR products were excised from a 0.8% agarose gel and purified using the GENECLEAN III kit (Q•BIOgene, BIO 101 Systems). The PCR products were directly sequenced at the Virginia Bioinformatics Institute Core Laboratory Facility with an automated DNA Sequencer. The PCR primer sequences were excluded from the resulting sequences. Only 269 bp of the resulting 386 bp partial helicase gene and 172 bp of the resulting 242 bp partial ORF2 gene sequences of these avian HEV isolates were used for comparison with the available corresponding regions of BLSV, swine and human HEVs. The sequences of avian HEV isolates from a healthy chicken and 14 avian HEV isolates from chickens with HS syndrome were analyzed and compared with the corresponding regions of the prototype avian HEV isolate, BLSV, and selected strains of swine and human HEVs by the MacVector computer program (Oxford Molecular Inc.). The percentages of nucleotide sequence identities among different HEV strains were determined. Phylogenetic analyses were conducted with the aid of the PAUP program (David L. Swofford, Smithsonian Institute, Washington DC, USA). The branch-and-bound search and mid-point rooting options with 1,000 replicates were used to generate the phylogenetic trees. Phylogenetic analyses were performed on two different genomic regions: a 269 bp fragment of the ORF 1 helicase gene, for which the sequence of BLSV and other isolates is also known, and a 172 bp fragment of the ORF2 capsid gene. 3.3.7 Experimental Infection of Young SPF Chickens with Avian HEV Isolates Recovered from A Healthy Chicken Flock To further confirm our field study, we attempted to experimentally infect SPF chickens with the PCR-positive materials from healthy chickens. Briefly, seven 1-week- 49 old SPF chickens (SPAFAS Inc., Norwich, CT) were each inoculated intravenously with 0.2 ml of positive fecal or serum material from each of the six field isolates (2553-26F, 2553-27F, 2553-28F, 2553-26S, 2553-27S and 2553-28S), respectively, recovered from the apparently healthy chicken flock, and each chicken was housed separately. One chicken (#3772) was not inoculated, and kept together with one inoculated chicken (#3771, inoculated with isolate 2553-28F) as a contact control. Weekly serum and fecal samples were collected from each chicken and tested by RT-PCR for viremia and fecal virus shedding. Serum samples were also tested by ELISA for seroconversion. 3.3.8 GenBank Accession Numbers The nucleotide sequences of the partial helicase and capsid gene regions of the avian HEV isolates reported in this paper have been deposited to the GenBank database with accession numbers from AY870808 to AY870831, and from AY871081 to AY871083. The GenBank accession numbers for the nucleotide sequences of the 11 avian HEV isolates as well as the prototype avian HEV, Australian chicken big liver and spleen disease virus (BLSV), and selected known representative strains of swine HEV and human HEV used in the sequence comparison and phylogenetic analyses are: CA077 (AF531908), CA242 (AF531898), CA518.5 (AY870832), CA697A (AF531907), CA697B (AF531906), CA697C (AF531903), CT090A (AF531905), CT690.2 (AF531899), NY449A (AF531902), WI318B (AF531901), WI966G (AF531900), prototype avian HEV (AY535004), BLSV Australia (Sequence is not available in GenBank database, and directly obtained from C. J. Payne), prototype swine HEV (AF082843), HEV US2 (AF060669), HEV Sar-55 (M80581), HEV Mexico (M74506) and HEV T1 (AJ272108). 3.4 Results 3.4.1 Subclinical Infection of Chickens by Avian HEV in A Commercial Chicken Farm Avian HEV isolates have thus far been genetically identified only from chickens with HS syndrome (Haqshenas et al., 2001b; Huang et al., 2002a). Detection of avian 50 HEV antibody in the majority of healthy chicken flocks in different geographic regions of the United States (Huang et al., 2002a) suggested that avian HEV infections are widespread, and are not just limited to chickens with HS syndrome. In order to genetically identify an avian HEV isolate from healthy chickens, we performed a prospective study in a healthy commercial chicken farm. All 14 chickens from this healthy farm were seronegative at 12 weeks of age when this prospective study began. The first chicken seroconverted at the age of 13 weeks in building no. 1, followed within a few weeks by seroconversion of chickens in other cages housed in the same building (Table 3.1). Chickens in two other buildings began to seroconvert at about 14-16 weeks of ages (Table 3.1). Most of the chickens in all three buildings seroconverted at about 17-19 weeks of ages. By 21 weeks of age, all the 14 study chickens had seroconverted and remained seropositive at the end of the study (30 weeks of age). However, none of the 14 chickens had any clinical sign of diseases consistent with HS syndrome. The course of antibody appearance in six representative chickens from this prospective study is presented in Fig. 3.1. The data from this prospective study indicate that avian HEV is enzoonotic in chicken flocks and spread subclinically among chickens. 3.4.2 Genetic Identification and Characterization of Avian HEV Isolates from A Healthy Chicken Flock The weekly or biweekly fecal and serum samples were tested by RT-PCR for avian HEV RNA. Viremia and fecal virus shedding were detected from the serum (124115S and 1241-16S) and fecal (1241-16F, 1229-16F and 1236-16F) samples of three healthy chickens (1241, 1229 and 1236) at 15 and 16 weeks of age, respectively, and from the serum and fecal samples of another healthy chicken (2553-26F, 2553-26S, 2553-27F and 2553-28F) for three consecutive weeks. The PCR products amplified from these healthy chickens were sequenced in two different regions: a 386 bp region in helicase gene and a 242 bp region in the capsid gene. The sequences obtained were compared to those of BLSV, swine, human and avian HEV strains. Sequence analyses revealed that the sequences of the helicase gene region of the avian HEV isolates recovered from fecal and serum samples of four healthy chickens (no. 1229, 1236, 1241 and 2553) within different weeks are 80-100% identical (Table 3.2). 51 However, they shared 86-97% nucleotide sequence identities with the prototype avian HEV and 70-97% sequence identities with avian HEV isolates recovered from chickens with HS syndrome. They also shared 73-78% identities with the Australian chicken BLSV and 51-59% sequence identities with selected known representative strains of swine and human HEVs (Table 3.2). Sequence analyses based on the 172 bp fragment of the ORF2 gene revealed similar results. The ORF2 gene sequences of the isolates recovered from the fecal and serum samples of the same healthy chicken within three different weeks (2553-26F, 2553-26S, 2553-27F, 2553-27S, 2553-28F and 2553-28S) are 99-100% identical (Table 3.3). They shared 90% nucleotide sequence identity with the prototype avian HEV and 75-95% sequence identities with avian HEV isolates from chickens with HS syndrome. They also shared 47-51% sequence identities with selected known representative strains of swine and human HEVs (Table 3.3). Phylogenetic analyses based on the partial ORF1 helicase gene and the partial ORF2 capsid gene indicated that the avian HEV isolates recovered from the fecal and serum samples of the same healthy chicken within three consecutive weeks clustered together (Fig. 3.2). The isolates from the other healthy chickens are also clustered in the same group. The avian HEV isolates from the healthy chickens are genetically related to the prototype avian HEV and other avian HEV isolates associated with HS syndrome. 3.4.3 Avian HEV Isolates from A Healthy Chicken Flock is Transmissible to Young SPF Chickens under Laboratory Conditions To determine if the virus isolates recovered from the healthy chicken flock in the prospective study can transmit to laboratory chickens, seven chickens, seronegative at the beginning of this experiment, were each inoculated with one of the six PCR-positive fecal or serum materials. The inoculated chickens began to seroconvert at about 12 weeks postinoculation (WPI). The first inoculated chicken (#3774) became seroconverted at 12 WPI. By 18 WPI, three of the four remaining inoculated chickens (except #3770) as well as the contact control chicken (housed together with the inoculated chicken #3771) had seroconverted. The OD405 values ranged from 0.314 to 0.629, and were below 1.00, which is in contrary to the chickens experimentally infected with the prototype avian 52 HEV (OD405 values usually 1.300-1.700). The course of seroconversion to IgG anti-avian HEV in the seven chickens is presented in Fig. 3.3. Viral RNA was also detected from the fecal (at 2 and 3 WPI), serum (at 3 WPI) as well as bile samples (3WPI) of two inoculated chickens (#3769 and #3773) (Table 3.4). The remaining chickens were necropsied at 21 WPI and no gross lesions in livers or spleens were found. A 471-bp fragment within the helicase gene region of avian HEV was amplified by RT-PCR from experimentally infected chickens. After excluding the PCR primer sequences, only 426 bp of the resulting 471 bp partial helicase gene sequences were used for comparison. The sequences were identical to those of the inoculum except for a single nucleotide change at position 34 (C-T) for one chicken (#3769) and at position 348 (C-T) for another chicken (#3773), which did not change the amino acid. This demonstrated that the viruses recovered from the experimentally infected chickens had originated from the inoculum. 3.4.4 The Capsid Gene of Avian HEV Isolates Recovered from Chickens with HS Syndrome in Different Geographic Regions of the United States is Heterogeneic It has been shown that the helicase gene of avian HEV isolates recovered from chickens with HS syndrome in different geographic regions varied considerably (Huang et al., 2002a). However, the extent of genetic variations of the important ORF2 capsid gene of avian HEV is not known since only one strain of avian HEV has thus far been characterized for its capsid gene (Haqshenas et al., 2001). By using RT-PCR, we successfully amplified the capsid gene region of 14 avian HEV isolates from the bile samples of chickens with HS syndrome in four different states. Sequence analyses revealed that these 14 avian HEV isolates from chickens with HS syndrome in different geographic regions shared 76-100% nucleotide sequence identities with each other, and 48-54% sequence identities with known swine and human HEV strains (Tables 3.2, 3.3). The nucleotide sequence identities of the ORF2 gene region for the isolates recovered from chickens in the same state ranged from 80 to 100% in California (CA077, CA242, CA518.3, CA518.5, CA697A, CA697B, CA697C, CA708 and CA 889), and 96 to 98% in Wisconsin (WI318B, WI966B and WI 966G). Even within the same farm, the partial 53 ORF2 gene of different avian HEV isolates varied from 93 to 100% nucleotide sequence identities among the three isolates in a California farm (CA697A, CA697B and CA697C), and 99% identity between the two isolates in another California farm (CA518.3 and CA518.5) (Tables 3.2, 3.3). Phylogenetic analyses of the ORF2 capsid gene region revealed that the avian HEV isolates from chickens with HS syndrome in different geographic regions are genetically heterogeneic. Minor branches, indicating heterogeneity, exist among avian HEV isolates regardless of their geographic origins. Avian HEV isolates from both healthy chickens and from chickens with HS syndrome are more distantly related to BLSV (Fig. 3.2). 3.5 Discussion Since avian HEV was discovered only about two years ago, little is known about its transmission and pathogenesis. Similar to swine and human HEVs, avian HEV is presumably transmitted through the fecal-oral route (Haqshenas et al., 2001, 2002; Huang et al., 2002a). Feces from infected chickens appear to be the major source of the virus. Chickens may get infected through direct contact with infected ones or through fecescontaminated feed or water. It is known that subclinical infection is the main outcome of swine HEV infection in pigs (Meng et al., 1997; Halbur et al., 2001; Meng, 2000a, b, 2003), and is also common for human HEV infections in humans (Purcell, 1996; Mast et al., 1997; Thomas et al., 1997; Meng et al., 2002). We have previously shown that the majority of chicken flocks in the United States are seropositive for avian HEV antibodies (Huang et al., 2002a), indicating that avian HEV infection is wide-spread among chickens. However, only sporadic cases of HS syndrome in chickens have been reported in the U.S., and so far avian HEV isolates have only been genetically identified from chickens with HS syndrome. Here we report for the first time the genetic identification and characterization of an avian HEV isolate from a clinically healthy chicken in a normal commercial chicken farm. The data from the prospective study confirmed our previous seroepidemiological study that avian HEV is enzootic in U.S. chicken flocks (Huang et al., 2002a), and provided convincing evidences that subclinical avian HEV infections occur in the 54 majority of chickens in the United States. We demonstrated that, under natural conditions, chickens became infected at about 3-4 months of age, and that viremia and fecal virus shedding occurred in some chickens prior to seroconversion and lasted only about 1-3 weeks. We speculate that the nature of subclinical infection in the majority of chickens is due to the relative low doses of virus that can be transmitted among chickens via fecal-oral route. It has been documented that human HEV infection in primates is dose-dependent: primates that received higher doses of human HEV developed biochemical and virological evidence of hepatitis, whereas primates that received lower doses had only subclinical infection, as evidenced by seroconversion to anti-HEV antibodies (Tsarev et al., 1994). This dose-dependant hypothesis may explain why there are only sporadic cases of HS syndrome in chickens, even though most chickens in the United States are infected by avian HEV (Huang et al., 2002a). The ORF2 capsid gene sequence of avian HEV is only known for one strain (Haqshenas et al., 2001). To determine the extent of genetic variations in the capsid gene of avian HEV, we amplified and sequenced the capsid gene region of 14 additional avian HEV isolates from chickens with HS syndrome in 4 different states (CA, WI, CT, and NY). Sequence and phylogenetic analyses revealed that the capsid gene sequences of avian HEV isolates from chickens with HS syndrome in different geographic regions of the United States are genetically heterogeneic. Avian HEV isolates showed considerable heterogeneity regardless of their geographic origins. This observation is consistent with reports on swine and human HEVs, which are also heterogeneic. Sequence analyses based on the ORF1 helicase gene and ORF2 capsid gene regions showed that the sequences of the avian HEV isolates recovered from healthy chickens within different weeks are 80-100% identical. However, they shared only 8697% nucleotide sequence identities with the prototype avian HEV and 70-97% sequence identities with avian HEV isolates recovered from chickens with HS syndrome. Phylogenetic analyses also revealed that the avian HEV isolates identified from healthy chickens clustered together (Fig. 3.2), and are genetically related to, but different from, the prototype avian HEV and avian HEV isolates recovered from chickens with HS syndrome. 55 In summary, the genetic identification of avian HEV isolates from a healthy chicken farm and the demonstration of subclinical avian HEV infection in chicken flocks in the United States further complicated the causal relationship between avian HEV infection and HS syndrome in chickens. Although we believe that, like swine and human HEVs, avian HEV infection is dose-dependent and only chickens infected with higher doses of the virus develop HS syndrome, we can not rule out the possibility that the avian HEV isolates identified from the healthy chickens may represent some avirulent strains and that the subclinical infections may be caused by the avirulent strains of avian HEV. Further studies are warranted to fully experimentally and genetically characterize these avian HEV isolates recovered from the healthy chickens. 3.6 Acknowledgements We would like to thank Dr. H. L. Shivaprasad and Dr. P. R. Woolcock for providing clinical samples; Dr. Mohamed Seleem for his expert assistance in protein purification. This project was supported by grants from the National Institute of Health (AI01653, AI46505, and AI50611), and from the U.S. Department of Agriculture National Research Initiatives Competitive Grant Program (NRI35204-12531). 56 3.7 References 1. Aggarwal, R. & Krawczynski, K. (2000). Hepatitis E: an overview and recent advances in clinical and laboratory research. Journal of Gastroenterology and Hepatology 15, 9-20. 2. Arankalle, V. A., Chadha, M. S., Tsarev, S. A., Emerson, S. U., Risbud, A. R., Banerjee, K. & Purcell, R. H. (1994). Seroepidemiology of water-borne hepatitis in India and evidence for a third enterically-transmitted hepatitis agent. Proceedings of the National Academy of Sciences, USA 91, 3428-3432. 3. Chandler, J. D., Riddell, M. A., Li, F., Love, R. J. & Anderson, D. A. (1999). Serological evidence for swine hepatitis E virus infection in Australian pig herds. Veterinary Microbiology 68, 95-105. 4. Clemente-Casares, P., Pina, S., Buti, M., Jardi, R., Martin, M., Bofill-Mas, S. & Girones, R. (2003). Hepatitis E virus epidemiology in industrialized countries. Emerging Infectious Diseases 9, 448-454. 5. Emerson, S. U., Zhang, M., Meng, X. J., Nguyen, H., Huang, Y. & Purcell, R. H. (2001). Recombinant hepatitis E virus genomes infectious for primates: importance of capping and discovery of a cis-reactive element. Proceedings of the National Academy of Sciences, USA 98, 15270-15275. 6. Erker, J. C., Desai, S. M., Schlauder, G. G., Dawson, G. J. & Mushahwar, I. K. (1999). A hepatitis E virus variant from the United States: molecular characterization and transmission in cynomolgus macaques. Journal of General Virology 80, 681-690. 7. Favorov, M. O., Kosoy, M. Y., Tsarev, S. A., Childs, J. E. & Margolis, H. S. (2000). Prevalence of antibody to hepatitis E virus among rodents in the United States. Journal of Infectious Diseases 184, 449-455. 8. Garkavenko, O., Obriadina, A., Meng, J., Anderson, D. A., Benard, H. J., Schroeder, B. A., Khudaakov, Y. E., Fields, H. A. & Croxson, M. C. (2001). Detection and characterization of swine hepatitis E virus in New Zealand. Journal of Medical Virology 65, 525-529. 9. Halbur, P. G., Kasorndorkbua, C., Gilbert, C., Guenette, D., Potters, M. B., Purcell, R. H., Emerson, S. U., Toth, T. E. & Meng, X. J. (2001). Comparative 57 pathogenesis of infection of pigs with hepatitis E viruses recovered from a pig and a human. Journal of Clinical Microbiology 39, 918-923. 10. Haqshenas, G. & Meng, X. J. (2001). Determination of the nucleotide sequences at the extreme 5’ and 3’ ends of swine hepatitis E virus genome. Archives of Virology 146, 2461-2467. 11. Haqshenas, G., Shivaprasad, H. L., Woolcock, P. R., Read, D. H. & Meng, X. J. (2001). Genetic identification and characterization of a novel virus related to human hepatitis E virus from chickens with hepatitis-splenomegaly syndrome in the United States. Journal of General Virology 82, 2449-2462. 12. Haqshenas, G., Huang, F. F., Fenaux, M., Guenette, D. K., Pierson, F. W., Larsen, C. T., Shivaprasad, H. L., Toth, T. E. & Meng, X. J. (2002). The putative capsid protein of the newly identified avian hepatitis E virus shares antigenic epitopes with that of swine and human hepatitis E viruses and chicken big liver and spleen disease virus. Journal of General Virology 83, 2201-2209. 13. Hsieh, S. Y., Meng, X. J., Wu, Y. H., Liu, S. T., Tam, A. W., Lin, D. Y. & Liaw, Y. F. (1999). Identity of a novel swine hepatitis E virus in Taiwan forming a monophyletic group with Taiwan isolates of human hepatitis E virus. Journal of Clinical Microbiology 37, 3828-3834. 14. Huang, C. C., Nguyen, D., Fernandez, J., Yun, K. Y., Fry, K. E., Bradley, D. W., Tam, A. W. & Reyes, G. R. (1992). Molecular cloning and sequencing of the Mexico isolate of hepatitis E virus (HEV). Virology 191, 550-558. 15. Huang, F. F., Haqshenas, G., Shivaprasad, H. L., Guenette, D. K., Woolcock, P. R., Larsen, C. T., Pierson, F. W., Elvinger, F., Toth, T. E. & Meng, X. J. (2002a). Heterogeneity and seroprevalence of the newly identified avian hepatitis E virus from chickens in the United States. Journal of Clinical Microbiology 40, 4197-4202. 16. Huang, F. F., Haqshenas, G., Guenette ,D. K., Halbur, P. G., Schommer, S., Pierson, F. W., Toth, T. E. & Meng, X. J. (2002b). Detection by RT-PCR and genetic characterization of field isoltes of swine hepatitis E virus from pigs in different geographic regions of the United States. Journal of Clinical Microbiology 40, 13261332. 17. Hussaini, S. H., Skidmore, S. J., Richardson, P., Sherratt, L. M., Cooper, B. T. & 58 O’Grady, J. G. (1997). Severe hepatitis E infection during pregnancy. Journal of Viral Hepatitis 4, 51-54. 18. Kabrane-Lazizi, Y., Fine, J. B., Elm, J., Glass, G. E., Higa, H., Diwan, A., Gibbs, Jr. C. J., Meng, X. J., Emerson, S. U. & Purcell, R. H. (1999). Evidence for widespread infection of wild rats with hepatitis E virus in the United States. American Journal of Tropical Medical Hygiene 61, 331-335. 19. Kasorndorkbua, C., Halbur, P. G., Guenette, D. K., Toth, T. E. & Meng, X. J. (2002). Use of a swine bioassay and a RT-PCR assay to assess the risk of transmission of swine hepatitis E virus in pigs. Journal of Virological Methods 101, 71-78. 20. McCrudden, R., O’Connell, S., Farrant, T., Beaton, S., Iredale, J. P. & Fine, D. (2000). Sporadic acute hepatitis E in the United Kingdom: an underdiagnosed phenomenon? Gut 46, 732-733. 21. Mast, E. E., Kuramoto, I. K., Favorov, M. O., Schoening, V. R., Burkholder, B. T., Shapiro, C. N. & Holland, P. V. (1997). Prevalence of and risk factors for antibody to hepatitis E virus seroreactivity among blood donors in Northern California. Journal of Infectious Diseases 176, 34-40. 22. Meng, X. J., Purcell, R. H., Halbur, P. G., Lehman, J. R., Webb, D. M., Tsareva, T. S., Haynes, J. S., Thacker, B. J. & Emerson, S. U. (1997). A novel virus in swine is closely related to the human hepatitis E virus. Proceedings of National Academy of Sciences, USA 94, 9860-9865. 23. Meng, X. J., Halbur, P. G., Shapiro, M.S., Govindarajan, S., Bruna, J. D., Mushahwar, I. K., Purcell, R. H. & Emerson, S. U. (1998). Genetic and experimental evidence for cross-species infection by the swine hepatitis E virus. Journal of Virology 72, 9714-9721. 24. Meng, X. J., Dea, S., Engle, R. E., Friendship, R., Lyoo, Y. S., Sirinnarumitr, T., Urairong, K., Wang, D., Wong, D., Yoo, D., Zhang, Y., Purcell, R. H. & Emerson, S. U. (1999). Prevalence of antibodies to the hepatitis E virus in pigs from countries where hepatitis E is common or is rare in the human population. Journal of Medical Virology 58, 297-302. 25. Meng, X. J. (2000a). Zoonotic and xenozoonotic risks of hepatitis E virus. Infectious Disease Revviews 2, 35-41. 59 26. Meng, X. J. (2000b). Novel strains of hepatitis E virus identified from humans and other animal species: is hepatitis E a zoonosis? Journal of Hepatology 33, 842-845. 27. Meng, X. J., Wiseman, B., Elvinger, F., Guenette, D. K., Toth, T. E., Engle, R. E., Emerson, S. U. & Purcell, R. H. (2002). Prevalence of antibodies to the hepatitis E virus in veterinarians working with swine and in normal blood donors in the United States and other countries. Journal of Clinical Microbiology 40, 117-122. 28. Meng, X. J. (2003). Swine hepatitis E virus: cross-species infection and risk in xenotransplantation. Current Topics in Microbiology and Immunology 278, 185216. 29. Nishizawa, T., Takahashi, M., Mizuo, H., Miyajima, H., Gotanda, Y. & Okamoto, H. (2003). Characterization of Japanese swine and human hepatitis E virus isolates of genotype IV with 99% identity over the entire genome. Journal of General Virology 84, 1245-1251. 30. Okamoto, H., Takahashi, M., Nishizawa, T., Fukai, K., Muramatsu, U. & Yoshikawa, A. (2001). Analysis of the complete genome of indigenous swine hepatitis E virus isolated in Japan. Biochemistry and Biophysics Research Communications 289, 929-936. 31. Payne, C. J., Ellis, T. M., Plant, S. L., Gregory, A. R. & Wilcox, G. E. (1999). Sequence data suggests big liver and spleen disease virus (BLSV) is genetically related to hepatitis E virus. Veterinary Microbiology 68, 119-125. 32. Pina, S., Buti, M., Cotrina, M., Piella, J. & Girones, R. (2000). HEV identified in serum from humans with acute hepatitis and in sewage of animal origin in Spain. Journal of Hepatology 33, 826-833. 33. Purcell, R. H. (1996). Hepatitis E virus. In Field Virology, 3rd edn, vol. 2, pp. 2831-2843. Edited by B. N. Fields, D. M. Knipe & P. M. Howley. Philadelphia: Lippincott-Raven. 34. Reyes, G. R. (1997). Overview of the epidemiology and biology of the hepatitis E virus. In Viral Hepatitis, pp. 239-258. Edited by R. A. Willson. New York: Marcel Dekker. 35. Riddell, C. (1997). Hepatitis-splenomegaly syndrome. In Diseases of Poultry, p. 1041. Edited by B. W. Calnek, H. J. Barnes, C. W. Beard, L. R. McDougald. & Y. M. 60 Saif. Ames, IA: Iowa State University Press. 36. Ritchie, S. J. & Riddell, C. (1991). “Hepatitis-splenomegaly” syndrome in commercial egg laying hens. Canadian Veterinary Journal 32, 500-501. 37. Schlauder, G. G., Dawson, G.J., Erker, J.C., Kwo, P.Y., Knigge, M. F., Smalley, D. L., Rosenblatt, J. E., Desai, S. M. & Mushahwar, I. K. (1998). The sequence and phylogenetic analysis of a novel hepatitis E virus isolated from a patient with acute hepatitis reported in the United States. Journal of General Virology 79, 447-456. 38. Schlauder, G. G., Desai, S. M., Zanetti, A. R., Tassopoulos, N. C. & Mushahwar, I. K. (1999). Novel hepatitis E virus (HEV) isolates from Europe: evidence for additional genotypes of HEV. Journal of Medical Virology 57, 243-251. 39. Shivaprasad, H. L. & Woolcock, P. R. (1995). Necrohemorrhagic hepatitis in broiler breeders. In Proceedings of the 44th Western Poultry Diseases Conference, 5-7 March, 1995, p. 6. Sacramento, CA, USA. 40. Sun, Z. F., Huang, F. F., Halbur, P. G., Schommer, S. K., Pierson, F. W., Toth, T. E. & Meng, X. J. (2003). Use of heteroduplex mobility assays (HMA) for presequencing screening and identification of variant strains of avian and swine hepatitis E viruses. Veterinary Microbiology 96, 165-176. 41. Takahashi, K., Iwata, K., Watanabe, N., Hatahara, T., Ohta, Y., Baba, K. & Mishiro, S. (2001). Full-genome nucleotide sequence of a hepatitis E virus strain that may be indigenous to Japan. Virology 287, 9-12. 42. Takahashi, K., Kang, J. H., Ohnishi, S. & Mishiro, S. (2002). Genetic heterogeneity of hepatitis E virus recovered from Japanese patients with acute sporadic hepatitis. Journal of Infections Diseases 185, 1342-1345. 43. Takahashi, M., Nishizawa, T., Miyajima, H., Gotanda, Y., Iita, T., Tsuda, F. & Okamoto, H. (2003a). Swine hepatitis E virus strains in Japan form four phylogenetic clusters comparable with those of Japanese isolates of human hepatitis E virus. Journal of General Virology 84, 851-862. 44. Takahashi, M., Nishizawa, T. & Okamoto, H. (2003b). Identification of a genotype III swine hepatitis E virus that was isolated from a Japanese pig born in 1990 and that is most closely related to Japanese isolates of human hepatitis E virus. Journal of Clinical Microbiology 41, 1342-1343. 61 45. Tei, S., Kitajima, N., Takahashi, K. & Mishiro, S. (2003). Zoonotic transmission of hepatitis E virus from deer to human beings. Lancet 362, 371-373. 46. Thomas, D. L., Yarbough, P. O., Vlahov, D., Tsarev, S. A., Nelson, K. E., Saah, A. J. & Purcell, R. H. (1997). Seroreactivity to hepatitis E virus in areas where the disease is not endemic. Journal of Clinical Microbiology 35, 1244-1247. 47. Tsarev, S. A., Emerson, S. U., Reyes, G. R., Tsareva, T. S., Legters, L. J., Malik, I. A., Iqbal, M. & Purcell, R. H. (1992). Characterization of a prototype strain of hepatitis E virus. Proceedings of National Academy of Sciences, USA 89, 559-563. 48. Tsarev, S. A., Tsareva, T. S., Emerson, S. U., Yarbough, P. O., Legters, L. J., Moskal, T. & Purcell, R. H. (1994). Infectivity titration of a prototype strain of hepatitis E virus in cynomolgus monkeys. Journal of Medical Virology 43, 135-142. 49. van der Poel, W. H, Verschoor, F., van der Heide, R., Herrera, M. I., Vivo, A., Kooreman, M. & de Roda Husman, A. M. (2001). Hepatitis E virus sequences in swine related to sequences in humans, the Netherlands. Emerging Infectious Diseases 7, 970-976. 50. Wang, Y., Ling, R., Erker, J. C., Zhang, H., Li, H., Desai, S., Mushahwar, I. K. & Harrison, T. J. (1999). A divergent genotype of hepatitis E virus in Chinese patients with acute hepatitis. Journal of General Virology 80, 169-177. 51. Wang, Y., Zhang, H., Ling, R., Li, H. & Harrison, T. J. (2000). The complete sequence of hepatitis E virus genotype 4 reveals an alternative strategy for translation of open reading frames 2 and 3. Journal of General Virology 81, 1675-1686. 52. Wang, Y. C., Zhang, H. Y., Xia, N. S., Peng, G., Lan, H. Y., Zhuang, H., Zhu, Y. H., Li, S. W., Tian, K. G., Gu, W. J., Lin, J. X., Wu, X., Li, H. M. & Harrison, T. J. (2002). Prevalence, isolation, and partial sequence analysis of hepatitis E virus from domestic animals in China. Journal of Medical Virology 67, 516-521. 53. Williams, T. P. E., Kasorndorkbua, C., Halbur, P. G., Haqshenas, G., Guenette, D. K., Toth, T. E. & Meng, X. J. (2001). Evidence of extrahepatic sites of replication of the hepatitis E virus in a swine model. Journal of Clinical Microbiology 39, 30403046. 62 54. Wu, J. C., Chen, C. M., Chiang, T. Y., Tsai, W. H., Jeng, W. J., Sheen, I. J., Lin, C. C. & Meng, X. J. (2002). Spread of hepatitis E virus among different-aged pigs: two- year survey in Taiwan. Journal of Medical Virology 66, 488-492. 55. Zanetti, A. R., Schlauder, G. G., Romano, L., Tanzi, E., Fabris, P., Dawson, G. J. & Mushahwar, I. K. (1999). Identification of a novel variant of hepatitis E virus in Italy. Journal of Medical Virology 57, 356-360. 63 12 13 14 15 16 17 19 21 26 27 29 30 Age (week 1226 1229 1230 1236 1241 2553 Chicken ID cutoff value is 0.300 OD405. 64 and remained seropositive at the end of the study, which was 30 weeks of age. The ELISA was 12 weeks of age. All six representative chickens had seroconverted by 21 weeks of age prospective study. All chickens were seronegative at the beginning of the experiment which Fig. 3.1. Seroconversion to avian HEV antibody in six representative chickens from the 0.0 0.2 0.4 0.6 0.8 1.0 1.2 1.4 A Fig. 3.2. 10 changes Swine HEV US HEV US2 HEV T1 HEV Mexico HEV Sar-55 BLSV Australia CA518.5 CA242 CT090A CA697C CA697B CA697A CA077 65 Avian HEV US 5 changes CA697A HEV T1 CA697B CA697C CT090 CA077 Swine HEV US HEV US2 HEV Sar-55 CA708A CA518.5 CA518.3 CA242 NY449 HEV Mexico CA889 Avian HEV US WI966G WI966B WI318B 2553-28S WI966G WI318B 2553-27S 2553-28F 2553-28F 2553-26S 2553-26F 2553-27F B 2553-27F 2553-26S 2553-26F Fig. 3.2. A. A phylogenetic tree based on the nucleotide sequences of a 269 bp partial helicase gene region of avian HEV isolates and Australian chicken big liver and spleen disease virus (BLSV), as well as selected known representative strains of swine and human HEVs. B. A phylogenetic tree based on the nucleotide sequences of a 172 bp partial ORF2 capsid gene region of avian HEV isolates and elected known representative strains of swine and human HEVs. The tree was constructed with the aid of the PAUP program. A branch and bound search with 1,000 replicates and a midpoint rooting option was used to construct the tree. A scale bar representing the numbers of character state changes is proportional to the genetic distance. The avian HEV isolates recovered from four healthy chickens in the prospective study are shown in boldface. Isolates identified from fecal material are annotated with letter F and isolates from serum material are annotated with letter S. 66 0 1 2 3 3769--2553-26F 3770--2553-27F 3771--2553-28F 3773--2553-26S 3774--2553-27S 3775--2553-28S 3772--Contact Inocula 5 6 7 8 10 12 14 16 18 20 21 Weeks Post-inoculation (WPI) 4 3769 3770 3771 3772 3773 3774 3775 Chicken ID 67 chicken (-♦ -) and #3773 chicken (-•-) were necropsied at 3 WPI. study. All chickens were seronegative at the beginning of the experiment. The ELISA cutoff value is 0.300 OD405. #3769 Fig. 3.3. Seroconversion to avian HEV antibody in six inoculated and one contact control chickens in the transmission 0 0.1 0.2 0.3 0.4 0.5 0.6 0.7 III II I Bldg No. – – – 1234 1235 1236 – – 1233 2553 – 1232 – – 1230 1241 – 1229 – – 1228 1238 – – 1226 1227 – 12 1225 Chicken ID – – – – – – – – – – – + – – 13 – – – – – – + – – – – + – – 14 – – – – – – + – + – – + – – 15 – – – + – + + + + – – + – – 16 68 – + – + – + + + + – + + – + 17 – + + + – + + + + + + + – + 19 + + + + + + + + + + + + + + 21 Age of chickens (weeks) + + + + + + + + + + + + + + 26 + + + + + + + + + + + + + + 27 + + + + + + + + + + + + + + 28 + + + + + + + + + + + + + + 29 Table 3.1. Seroconversion to IgG avian HEV antibody in healthy chickens from a normal commercial chicken farm: a prospective study + + + + + + + + + + + + + + 30 US2 Sar-55 Mexico T1 BLSV Swine HEV Avian HEV WI966G WI318B NY449A CT690.2 CT090A CA697C CA697B CA697A CA518.5 CA077 CA242 2553-28F 2552-27F 2553-26S 2553-26F 2553-26S 100 100 2553-27F 100 100 2553-28F 100 100 81 CA077 81 81 81 83 CA242 85 83 83 83 81 CA518.5 86 95 81 81 81 79 CA697A 86 97 85 79 79 79 79 CA697B 100 86 97 85 79 79 79 79 CA697C 100 100 86 97 85 79 79 79 83 CT090A 69 93 93 93 86 93 86 83 83 83 77 CT690.2 83 80 80 80 85 81 82 77 77 77 82 NY449A 82 99 94 94 94 86 94 87 82 82 82 97 WI318B 82 77 83 79 79 79 83 81 84 97 97 97 96 WI966G 96 81 78 81 78 78 78 81 79 84 96 96 96 86 Avian HEV 86 87 76 79 81 79 79 79 82 80 81 86 86 86 75 BLSV 76 76 76 78 81 75 75 75 75 76 75 76 75 75 75 54 Swine HEV 56 53 55 54 57 56 54 55 55 55 55 55 56 54 54 54 53 US2 56 92 54 54 54 57 55 53 55 55 55 55 55 56 53 53 53 54 Sar-55 74 55 77 55 55 55 54 53 54 55 55 55 55 55 55 54 54 54 72 79 55 76 55 53 53 59 58 56 56 56 56 55 57 55 52 52 52 52 Mexico 2553-26F Table 3.2. Pairwise sequence comparison of the helicase gene region of different avian HEV isolates, BLSV and selected known representative strains of swine and human HEVs 76 76 73 51 76 52 51 52 55 57 53 52 52 52 52 52 53 51 51 51 51 T1 2553-26F 2553-26S 2553-27F 2553-27S 2553-28F 2553-28S CA077 CA242 CA518.3 CA518.5 CA697A CA697B CA697C CA708A CA889 CT090 NY449 WI318B WI966B WI966G Avian HEV Swine HEV US2 Sar-55 Mexico T1 2553-26S 100 2553-27F 100 100 2553-27S 100 100 100 2553-28F 99 99 99 99 2553-28S 100 100 100 100 99 CA077 79 79 79 79 79 79 CA242 76 76 76 76 75 76 83 CA518.3 76 76 76 76 75 76 84 95 CA518.5 76 76 76 76 75 76 84 95 99 CA697A 79 79 79 79 79 79 94 86 86 86 CA697B 70 78 78 78 78 77 78 94 80 84 84 93 CA697C 78 78 78 78 77 78 94 80 84 84 93 100 CA708A 76 76 76 76 75 76 84 95 99 100 86 84 84 CA889 89 89 89 89 88 89 83 81 83 83 81 81 81 83 CT090 77 77 77 77 77 77 94 80 83 83 94 99 99 83 80 NY449 80 80 80 80 79 80 94 84 85 85 97 93 93 85 82 94 WI318B 95 95 95 95 95 95 79 77 77 77 80 78 78 77 88 77 79 WI966B 95 95 95 95 95 95 78 77 79 79 79 78 78 79 88 77 79 97 WI966G 95 95 95 95 95 95 77 76 77 77 79 78 78 77 88 77 79 96 98 Avian HEV 90 90 90 90 90 90 79 78 81 81 80 79 79 81 91 78 80 90 90 91 51 51 51 51 51 51 51 51 50 49 51 51 51 49 49 50 51 50 51 51 51 Swine HEV 2553-26F Table 3.3. Pairwise sequence comparison of the ORF2 capsid gene region of different avian HEV isolates and selected known representative strains of swine and human HEVs US2 51 51 51 51 50 51 51 54 52 51 51 50 50 51 51 49 50 49 50 50 50 88 Sar-55 47 47 47 47 47 47 51 51 51 51 50 51 51 51 48 52 49 48 49 49 50 79 75 Mexico 47 47 47 47 47 47 49 48 48 48 48 48 48 48 52 49 48 48 48 48 50 72 69 74 50 50 50 50 50 50 49 51 50 50 50 49 49 50 50 50 50 48 50 51 48 75 77 73 72 T1 3769 3770 3771 3773 3774 3775 3772 Inocula 2553-26F 2553-27F 2553-28F 2553-26S 2553-27S 2553-28S Contact control a -/- -/- -/- -/- -/- -/- -/- 0 -/- -/- -/- -/- -/- -/- -/- 1 -/- -/- -/- -/+ -/- -/- -/+ 2 -/- -/- -/- +/+ -/- -/- -/+ 3 b b -/- -/- -/- -/- -/- 4 NA/- NA/- NA/- NA/- c NA /- 5 b 71 -/- -/- -/- -/- -/- 6 NA/- NA/- NA/- NA/- NA/- 7 -/- -/- -/- -/- -/- 8 Weeks post-inoculation (WPI) Viremia / fecal virus shedding; -, negative; +, positive Two chickens necropsied at 3 WPI, bile samples were also positive for both chickens c NA, serum sample not available at this time point a ID No. -/- -/- -/- -/- -/- 10 -/- -/- -/- -/- -/- 12 NA/- NA/- NA/- NA/- NA/- 15 Table 3.4. Detection of avian HEV RNA in serum and fecal samples of chickens experimentally inoculated with avian HEV isolates recovered from a healthy chicken flock -/- -/- -/- -/- -/- 18 -/- -/- -/- -/- -/- 21 CHAPTER IV Generation and Infectivity Titration of An Infectious Stock of Avian Hepatitis E Virus (HEV) in Chickens and Cross-Species Infection of Turkeys with Avian HEV Z. F. Sun, C. T. Larsen, F. F. Huang, P. Billam, F. W. Pierson, T. E. Toth, and X. J. Meng Journal of Clinical Microbiology, 2004 Jun;42(6):2658-2662. Modified and re-printed with permission. 4.1 Abstract Avian hepatitis E virus (avian HEV), a novel virus discovered from chickens with hepatitis-splenomegaly (HS) syndrome in the United States, is genetically and antigenically related to human HEV. In order to further characterize avian HEV, an infectious viral stock with a known infectious titer must be generated as HEV can not be propagated in vitro. Bile and feces collected from specific-pathogen-free (SPF) chickens experimentally infected with avian HEV were used to prepare an avian HEV infectious stock as a 10% suspension of positive fecal and bile samples in PBS buffer. The infectivity titer of this infectious stock was determined by inoculating one-week old SPF chickens intravenously with 200 µl each of the serial 10-fold dilutions (10-2-10-6) of the avian HEV stock (two chickens per dilution). All chickens inoculated with 10-2-10-4 dilutions, one of the two chickens inoculated with 10-5 dilution, but not those two inoculated with 10-6 dilution of the infectious stock, seroconverted to avian HEV at 4 weeks post-inoculation (WPI). Two serologically negative contact control chickens housed together with the 10-2 dilution-inoculated chickens also seroconverted at 8 WPI. Viremia and fecal virus shedding were detected variably in chickens inoculated with 102 -10-5 dilutions but not in those inoculated with 10-6 dilution. The infectivity titer of the infectious avian HEV stock was determined to be 5 x 104.5 50% chicken infectious doses 72 (CID50) per ml. Eight one-week old turkeys were intravenously inoculated with 104.5 CID50 of avian HEV, and another group of nine birds were un-inoculated as controls. The inoculated turkeys seroconverted at 4-8 WPI. Viremia was detected at 2-6 WPI, and fecal virus shedding at 4-7 WPI in inoculated turkeys. A serologically negative contact control turkey housed together with the inoculated ones also became infected through direct contact. This is the first demonstration of cross-species infection by avian HEV. 73 4.2 Introduction Hepatitis E virus (HEV), the causative agent of hepatitis E, is an important human pathogen (1-2, 23-24, 26, 34-35). HEV is a positive-sense, single-strand, non-enveloped RNA virus. The genome of HEV is about 7.2 kb and contains three open reading frames (ORFs) (23-24, 26). Hepatitis E is primarily transmitted through the fecal-oral route with an incubation period of about 15-60 days. The mortality rate is generally low (about 1%), however, it can reach up to 15-25% among infected pregnant women (7, 23-24). HEV is a public health concern in many developing countries, however, sporadic cases of acute hepatitis E were also reported in many industrialized countries including the United States (3, 13-14, 17, 20, 22, 26, 31, 33, 37). Swine HEV, the first animal strain of HEV, was identified and characterized from a pig in the United States in 1997 (15). Many swine HEV isolates have since been identified worldwide and shown to be genetically close-related to genotypes 3 and 4 strains of human HEVs (5, 11, 19-20, 29, 33, 36-37). Recently, avian HEV, another animal strain of HEV, was identified from chickens with hepatitis-splenomegaly (HS) syndrome in the United States. Avian HEV was also demonstrated to be genetically and antigenically related to the known strains of human and swine HEVs (8-9). HS syndrome was first reported during 1991 in western Canada and then in the United States. The disease is characterized by increased mortality in broiler breeder and laying chickens of 30-72 weeks of age with up to 20% drop in egg production. Regressive ovaries, red fluid in the abdomen, enlarged liver and spleen were often seen in infected chickens with histological changes of hepatic necrosis and hemorrhage (25). Avian HEV has been genetically identified from chickens with HS syndrome as well as from healthy chickens (8, 10, 28). Avian HEV shares approximately 50-60% nucleotide sequence identities with known human and swine HEVs, and approximately 80% sequence identity with the Australian chicken big liver and spleen disease virus (BLSV) (8-10, 21). Cross-species infection of swine and human HEVs has been demonstrated as a human HEV infected SPF pigs, and a swine HEV infected non-human primates (6, 16). Anti-HEV antibodies have also been detected in many animal species, and hepatitis E is considered as a zoonosis (4, 12, 17-18, 30). The objectives of this study are to generate an infectious stock of avian HEV, to determine the infectivity titer of this viral stock in young specific-pathogenfree (SPF) chickens, and to attempt to experimentally infect SPF turkeys with avian HEV. 74 4.3 Materials and Methods 4.3.1 Virus Avian HEV used in the study was originally recovered from a bile sample of a naturally infected chicken with HS syndrome (8). Due to the limited amount of original avian HEV material, the virus was first amplified in 1-week-old SPF chickens (SPAFAS Inc., Norwich, CT) by intravenous inoculation of 0.1 ml of a 1:100 diluted original bile sample containing avian HEV. A positive fecal sample collected at 28 days post-inoculation (DPI) from an infected young SPF chicken was used to prepare a 10% fecal suspension in phosphate buffered saline (PBS). The genomic equivalence (GE) titer of avian HEV in the 10% fecal suspension is 104 GE / ml, and this fecal suspension of avian HEV was used to generate an infectious stock of avian HEV. 4.3.2 Primer Design PCR primers used for the detection of avian HEV from fecal, serum and bile samples of the experimentally inoculated SPF chickens and turkeys were based on the ORF1 helicase gene region (8, 10, 27). The primer sequences are: external primer set, AHEV F-1/S: 5’GAGCTTGTGAAGGGCGTTGAGG-3’; AHEV R-1/S: 5’ACCCAAGATCAACGGCGCTC-3’; internal primer set, AHEV F-2/S: 5’CGGCAAGTCGTCGTCTGTTGACCAT-3’; AHEV R-2/S: 5’CCCCCAGCATAACAACATCGCGC-3’. The sizes of expected PCR products for the first and second rounds were 359 bp and 221 bp, respectively. 4.3.3 RNA Extraction and RT-PCR RNA was extracted with TriReagent (Molecular Research Center, Inc.) from 100 µl of fecal, serum or bile samples from chickens or turkeys. Total RNA was resuspended in 12.25 µl of DNase-free, RNase-free, and proteinase-free water (Invitrogen). Reverse transcription was performed at 42°C for 60 min in the presence of a master mix consisting of 12.25 µl of total RNA, 0.25 µl of Superscript II reverse transcriptase (Invitrogen), 1 ul of 10 µM antisense primer, 0.5 µl of RNase inhibitor, 1 µl of 0.1M dithioteritol, 4 µl of 5x RT buffer, and 1 µl of 75 10 mM dNTPs. The resulting cDNA was amplified by a nested RT-PCR, using AmpliTaq Gold DNA polymerase (Applied Biosystems). The PCR reaction parameters were 95°C for 9 min, followed by 39 cycles of amplification at 94°C for 1 min for denaturation, 52°C for 1 min for annealing, 72°C for 1.5 min for extension, followed by a final incubation period at 72°C for 7 min. The PCR products were examined on a 0.8% agarose gel. PCR products from selected chickens and turkeys were sequenced to confirm the identity of the virus recovered from experimentally infected birds (10). 4.3.4 ELISA to Detect Anti-HEV Antibody in Chickens and Turkeys A truncated recombinant ORF2 capsid protein of avian HEV was expressed in E. coli strain BL21StarTM (DE3)pLysS (Invitrogen) and purified using the BugBuster His-Bind Purification Kit (Novagen) as previously described (9-10, 28). The purified avian HEV ORF2 protein was used as the antigen to standardize an ELISA to detect avian HEV antibodies in chickens and turkeys as described previously (10). Briefly, the purified antigen was coated onto 96-well flat bottom microtiter plates (Thermo Labsystems). A horseradish peroxidase (HRP)-conjugated rabbit anti-chicken IgG (Sigma) or a HRP-conjugated goat anti-turkey IgG (KPL) was used as the secondary antibody for chicken and turkey sera, respectively. The cutoff values for these assays were set conservatively at 0.30 as previously described (10, 28). Sera from serologically negative SPF chickens and SPF turkeys were used as negative controls, and convalescent sera from SPF chickens experimentally infected with avian HEV were included as positive controls. All sera were tested at least twice. 4.3.5 Generation of An Infectious Stock of Avian HEV Since HEV can not be propagated in cell culture, an infectious stock of avian HEV must be generated by using live animals. Ten 60-week-old SPF chickens (SPAFAS Inc., Norwich, CT) were each intravenously inoculated with approximately 103 GE of avian HEV (0.1 ml of the 10% fecal suspension) and monitored for 56 days. Feces were collected from all the inoculated chickens every 4 days. Two birds were each necropsied at 12, 18, and 22 DPI, respectively, and the remaining ones at 56 DPI. During each necropsy, bile and feces were 76 collected from each chicken. Fecal and bile samples were tested by RT-PCR for the presence of avian HEV RNA. 4.3.6 Infectivity Titration of the Avian HEV Stock in Young SpecificPathogen-Free (SPF) Chickens The infectivity titer of the virus stock was determined in young SPF chickens. Briefly, thirty 1-week-old SPF chickens (SPAFAS Inc., Norwich, CT) were randomly assigned into ten isolators of three each. The avian HEV stock was serially diluted 10-fold from 10-2 to 10-6 in PBS buffer. Each of the five dilutions was inoculated intravenously into two young SPF chickens (200 µl per chicken). Each inoculated chicken was housed in a separate isolator together with two serologically negative contact control chickens to assess the nature of avian HEV spread by direct contact. Bi-weekly serum samples from each chicken were tested by ELISA for seroconversion. Bi-weekly serum and weekly fecal samples from each chicken were tested by RT-PCR for viremia and fecal virus shedding. The chickens were monitored for a total of 12 weeks for evidence of avian HEV infection. The infectivity titer of the avian HEV stock was calculated as 50% chicken infectious dose (CID50) per ml of the virus inoculum. 4.3.7 Attempt to Experimentally Infect Young SPF Turkeys with Avian HEV from A Chicken To determine if avian HEV can infect across species, eighteen 1-week-old SPF turkeys (British United Turkeys of America, Lewisburg, WV) were randomly assigned into two groups of nine each. A 200 µl of the avian HEV infectious stock (104.5 CID50) was inoculated intravenously into each of the eight turkeys in one group, and the remaining turkey in this group was not inoculated as a contact control. The nine turkeys in the second group were uninoculated as controls. Bi-weekly serum samples from each turkey were collected and tested by ELISA for seroconversion to avian HEV antibody. Bi-weekly serum and weekly fecal samples from each turkey were tested by RT-PCR for viremia and fecal virus shedding. 77 4.3.8 GenBank Accession Numbers The nucleotide sequences of the partial helicase gene region of the avian HEV isolates reported in this paper have been deposited to the GenBank database with accession numbers from AY871084 to AY871090. 4.4 Results 4.4.1 Generation of An Infectious Stock of Avian HEV Due to the limited amount of original bile sample containing avian HEV, we first amplified the virus in a 1-week-old SPF chicken. A 10% suspension of a positive fecal material collected from an infected young SPF chicken was prepared. This 10% fecal suspension of avian HEV, which contains only approximately 104 GE / ml of avian HEV, was then used to intravenously inoculate ten 60-week-old SPF chickens to generate an infectious stock of avian HEV. Bile and feces collected during necropsies at 12, 18, and 22 DPI were positive for avian HEV RNA. Positive feces and bile samples from the 4 SPF chickens necropsied at 18 and 22 DPI were pooled to make a 10% suspension in PBS as an infectious stock of avian HEV. The viral stock was then aliquoted and stored at -80°C for further study. 4.4.2 Determination of the Infectivity Titer of An Avian HEV Stock In Vivo Seroconversion in the inoculated chickens was used as the end point for the calculation of the infectivity titer of the virus stock (Table 4.1). Both chickens inoculated with the highest amount of viruses at 10-2 dilution seroconverted to avian HEV at 4 WPI. Two of the serologically negative contact control chickens housed in the same isolator as one of the two inoculated with 10-2 dilution also seroconverted to avian HEV 4 weeks after the inoculated one had seroconverted. The chickens inoculated with 10-3 or 10-4 dilution, also seroconverted. However, none of the contact control chickens in these two groups seroconverted. Only one of the two 10-5 dilution inoculated chickens seroconverted at 4 WPI. The chickens inoculated with the lowest amount of viruses at 10-6 dilution remained negative for avian HEV antibodies, viremia, or fecal virus shedding (Table 4.1). Since each chicken received 200 µl of the virus 78 stock, the infectious titer of the virus stock was therefore calculated as 5 x 104.5 50% chicken infectious dose (CID50) per ml. 4.4.3 Subclinical Infection of Young Chickens by Avian HEV in A DoseDependent Manner All chickens inoculated with 10-2 or 10-3 dilution had transit viremia, and lasted approximately 1 week (Table 4.2). The viremia in one (#5357) of the two chickens received a 10-2 dilution lasted for 11 weeks. Avian HEV RNA was also detected from the bile sample of this chicken collected at 12 WPI during necropsy. Two of the contact control chickens in the 10-2 dilution group were also viremic but occurred at 6-8 weeks post-inoculation. Chickens inoculated with a 10-4, 10-5 or 10-6 dilution did not develop viremia (Table 4.2). All chickens inoculated with a 10-2, 10-3 or 10-4 dilution shed avian HEV in the feces for 1-2 weeks (Table 4.2). Like viremia, one (#5357) of the two chickens inoculated with a 10-2 dilution had fecal virus shedding lasting for 12 weeks. Two of the contact control chickens in the 10-2 dilution group also shed virus in feces for 1 or 3 weeks at 6-8 weeks postinoculation. One of the two chickens inoculated with a 10-5 dilution also had a transit fecal virus shedding. None of the chickens in the 10-6 dilution group had detectable virus RNA in the feces (Table 4.2). 4.4.4 Cross-Species Infection of SPF Turkeys with Avian HEV from A Chicken Since swine HEV was shown to infect across species (16-17, 19), we conducted this study to determine if the virus from a chicken can cross species barriers and infect young SPF turkeys. All 18 turkeys were seronegative at the beginning of this study. The first inoculated turkey (#2872) became seroconverted at 4 WPI (Table 4.3). By 8 WPI, all 4 remaining inoculated turkeys as well as the contact control turkey (housed together with the inoculated group) had seroconverted to avian HEV antibody (Table 4.3). No seroconversion or viral RNA was detected from the un-inoculated control turkeys. The course of seroconversion to avian HEV antibody in 7 representative turkeys from this cross-species 79 infection study is presented in Fig. 4.1. Two turkeys (#2788 and #2875) in the inoculated group died of anaphylaxis within 48 hours post-inoculation and another two (#2776 and #2790) died prior to seroconversion during a bi-weekly blood collection process at 2 WPI. No gross pathological lesions attributable to avian HEV infection were found during necropsies of the dead birds. The remaining turkeys in this group were necropsied at 12 WPI and no gross lesions in livers or spleens were found. Viremia was first detected from an inoculated turkey (#2872) at 2 WPI, followed by two other inoculated turkeys at 4 (#2791) and 6 WPI (#2873). Viral RNA was also detected from the bile sample of one turkey (#2791) collected at 11 WPI during necropsy. Fecal virus shedding was also detected variably from inoculated turkeys (Table 4.4). None of the control turkeys had any viral RNA in their serum, fecal and bile samples. A 221-bp fragment within the helicase gene region of avian HEV was amplified by RT-PCR from experimentally infected turkeys. After excluding the PCR primer sequences, only 173 bp of the resulting 221 bp partial helicase gene sequences were used for comparison. The sequences were identical to that of the inoculum except for four nucleotide changes in one turkey (#2791) at positions 72 (C-T), 78 (T-C), 99 (C-A) and 108 (T-C) from the fecal material at 7 WPI, and three nucleotide changes in the same turkey (#2791) at positions 72 (C-T), 78 (T-C) and 108 (T-C) from the bile at 11 WPI, which did not change the amino acid. No nucleotide change was detected from serum sample of the same turkey (#2791) at 6 WPI, and from fecal materials of other turkeys (#2792 at 6 WPI and #2873 at 5 and 6 WPI) compared to the original inoculum. This indicated that the viruses recovered from the experimentally infected turkeys had originated from the inoculum. 4.5 Discussion In this study, an infectious stock of avian HEV was generated and its infectivity titer was determined in SPF chickens. In the absence of a cell culture system, this standardized infectious stock of avian HEV will be very useful for future characterization of avian HEV pathogenesis and replication. The infectivity titration experiment also showed that young SPF chickens can be easily infected via intravenous route of inoculation of avian HEV. Viremia and fecal virus shedding are transit with the exception of one inoculated chicken, which lasted for more than 10 weeks. The co-existence of anti-avian HEV antibody and 80 viremia in this particular bird suggested that persistent infection of avian HEV might exist under some unknown conditions. The appearance of viremia and fecal virus shedding is apparently dose-dependent as virus shedding in blood and feces appeared earlier in chickens inoculated with higher dose than in those inoculated with lower dose. This observation is consistent with the infectivity titration results in monkeys inoculated with human HEV (32). We have previously shown that swine HEV infected non-human primates and human HEV infected pigs (6, 16). Therefore, after obtaining a standard infectious stock of avian HEV, we attempted to experimentally infect another avian species, turkeys, with avian HEV from a chicken. The inoculated turkeys seroconverted to avian HEV antibody at 4-8 WPI, although not all infected turkeys had viremia or fecal virus shedding. This suggested that the avian HEV from chickens may replicate at a lower level in turkeys. The contact control turkey housed together with the inoculated ones seroconverted but viremia and fecal virus shedding were not detected in this contact bird. The virus recovered from experimentally infected turkeys was sequenced for a 173 bp region within the helicase gene and was confirmed to originate from the inoculum. We noticed that there are 3-4 nucleotide changes (but no amino acid change) within the selected gene region of the viruses identified from the fecal and bile samples of one inoculated turkey, this may be necessary for this chicken virus to get adapted to its new host (turkeys), or indicate the presence of a quasispecies. The demonstrated ability of cross-species infection by avian HEV suggested that avian HEV may infect species other than chickens under field conditions. Further studies are warranted to assess the range of host susceptibility of avian HEV and its potential zoonotic risk. 4.6 Acknowledgements This project was supported in part by grants from the National Institute of Health (AI01653, AI46505, and AI50611), and from the U. S. Department of Agriculture National Research Initiative Competitive Grant Program (NRI 35204-12531). We would like to thank Mr. Denis Guenette for his technical assistance. 81 4.7 References 1. Aggarwal, R., and K. Krawczynski. 2000. Hepatitis E: an overview and recent advances in clinical and laboratory research. J. Gastroenterol. Hepatol. 15:9-20. 2. Arankalle, V. A., M. S. Chadha, S. A. Tsarev, S. U. Emerson, A. R. Risbud, K. Banerjee, and R. H. Purcell. 1994. Seroepidemiology of water-borne hepatitis in India and evidence for a third enterically-transmitted hepatitis agent. Proc. Natl. Acad. Sci. USA 91:3428-3432. 3. Erker, J. C., S. M. Desai, G. G. Schlauder, G. J. Dawson, and I. K. Mushahwar. 1999. A hepatitis E virus variant from the United States: molecular characterization and transmission in cynomolgus macaques. J. Gen. Virol. 80:681-690. 4. Favorov, M. O., M. Y. Kosoy, S. A. Tsarev, J. E. Childs, and H. S. Margolis. 2000. Prevalence of antibody to hepatitis E virus among rodents in the United States. J. Infect. Dis. 184:449-455. 5. Garkavenko, O., A. Obriadina, J. Meng, D. A. Anderson, H. J. Benard, B. A. Schroeder, Y. E. Khudaakov, H. A. Fields, and M . C. Croxson. 2001. Detection and characterization of swine hepatitis E virus in New Zealand. Journal of Medical Virology 65:525-529. 6. Halbur, P. G., C. Kasorndorkbua, C. Gilbert, D. K. Guenette, M. B. Potters, R. H. Purcell, S. U. Emerson, T. E. Toth, and X. J. Meng. 2001. Comparative pathogenesis of infection of pigs with hepatitis E viruses recovered from a pig and a human. J. Clin. Microbiol. 39:918-923. 7. Hamid, S. S., S. M. Jafri, H. Khan, H. Shah, Z. Abbas, and H. Fields. 1996. Fulminant hepatic failure in pregnant women: acute fatty liver or acute viral hepatitis? J. Hepatol. 25:20-27. 8. Haqshenas, G., H. L. Shivaprasad, P. R. Woolcock, D. H. Read, and X. J. Meng. 2001. Genetic identification and characterization of a novel virus related to human hepatitis E virus from chickens with hepatitis-splenomegaly syndrome in the United States. J. Gen. Virol. 82:2449-2462. 9. Haqshenas, G., F. F. Huang, M. Fenaux, D. K. Guenette, F. W. Pierson, C. T. Larsen, H. L. Shivaprasad, T. E. Toth, and X. J. Meng. 2002. The putative capsid protein of the newly identified avian hepatitis E virus shares antigenic epitopes with 82 that of swine and human hepatitis E viruses and chicken big liver and spleen disease virus. J. Gen. Virol. 83:2201-2209. 10. Huang, F. F., G. Haqshenas, H. L. Shivaprasad, D. K. Guenette, P. R. Woolcock, C. T. Larsen, F. W. Pierson, F. Elvinger, T. E. Toth, and X. J. Meng. 2002. Heterogeneity and seroprevalence of the newly identified avian hepatitis E virus from chickens in the United States. J. Clin. Microbiol. 40:4197-4202. 11. Huang, F. F., G. Haqshenas, D. K. Guenette , P. G. Halbur, S. Schommer, F. W. Pierson, T. E. Toth, and X. J. Meng. 2002. Detection by RT-PCR and genetic characterization of field isoltes of swine hepatitis E virus from pigs in different geographic regions of the United States. J. Clin. Microbiol. 40:1326-1332. 12. Kabrane-Lazizi, Y., J. B. Fine, J. Elm, G. E. Glass, H. Higa, A. Diwan, C. J. Gibbs, Jr., X. J. Meng, S. U. Emerson, and R. H. Purcell. 1999. Evidence for wide-spread infection of wild rats with hepatitis E virus in the United States. Am. J. Trop. Med. Hyg. 61:331-335. 13. Mast, E. E., I. K. Kuramoto, M. O. Favorov, V. R. Schoening, B. T. Burkholder, C. N. Shapiro, and P. V. Holland. 1997. Prevalence of and risk factors for antibody to hepatitis E virus seroreactivity among blood donors in Northern California. J. Infec. Dis. 176:34-40. 14. McCrudden, R., S. O’Connell, T. Farrant, S. Beaton, J. P. Iredale, and D. Fine. 2000. Sporadic acute hepatitis E in the United Kingdom: an underdiagnosed phenomenon? Gut 46:732-733. 15. Meng, X. J., R. H. Purcell, P. G. Halbur, J. R. Lehman, D. M. Webb, T. S. Tsareva, J. S. Haynes, B. J. Thacker, and S. U. Emerson. 1997. A novel virus in swine is closely related to the human hepatitis E virus. Proc. Natl. Acad. Sci. USA 94:9860-9865. 16. Meng, X. J., P. G. Halbur, M.S. Shapiro, S. Govindarajan, J. D. Bruna, I. K. Mushahwar, R. H. Purcell, and S. U. Emerson. 1998. Genetic and experimental evidence for cross-species infection by the swine hepatitis E virus. J. Virol. 72:9714-9721. 17. Meng, X. J. 2000. Novel strains of hepatitis E virus identified from humans and other animal species: is hepatitis E a zoonosis? J. Hepatol. 33:842-845. 83 18. Meng, X. J., B. Wiseman, F. Elvinger, D. K. Guenette, T. E. Toth, R. E. Engle, S. U. Emerson, and R. H. Purcell. 2002. Prevalence of antibodies to the hepatitis E virus in veterinarians working with swine and in normal blood donors in the United States and other countries. J. Clin. Microbiol. 40:117-22. 19. Meng, X. J. 2003. Swine hepatitis E virus: cross-species infection and risk in xenotransplantation. Curr. Topics Microbiol. Immunol. 278:185-216. 20. Nishizawa, T., M. Takahashi, H. Mizuo, H. Miyajima, Y. Gotanda, and H. Okamoto. 2003. Characterization of Japanese swine and human hepatitis E virus isolates of genotype IV with 99% identity over the entire genome. J. Gen. Virol. 84:1245-1251. 21. Payne, C. J., T. M. Ellis, S. L. Plant, A. R. Gregory, and G. E. Wilcox. 1999. Sequence data suggests big liver and spleen disease virus (BLSV) is genetically related to hepatitis E virus. Vet. Microbiol. 68:119-125. 22. Pina, S., M. Buti, M. Cotrina, J. Piella, and R. Girones. 2000. HEV identified in serum from humans with acute hepatitis and in sewage of animal origin in Spain. J. Hepatol. 33:826-833. 23. Purcell, R. H. 1996. Hepatitis E virus, p. 2831-2843. In B. N. Fields, D. M. Knipe, and P. M. Howley, et al. (ed.), Fields virology, 3rd ed., vol 2. Lippincott-Raven Publishers, Philadelphia, Pa. 24. Reyes, G. R. 1997. Overview of the epidemiology and biology of the hepatitis E virus, p. 239-258. In R. A. Willson (ed.), Viral hepatitis. Marcel Dekker, Inc., New York, N. Y. 25. Riddell, C. 1997. Hepatitis-splenomegaly syndrome, p. 1041. In B. W. Calnek, H. J. Barnes, C. W. Beard, et al. (ed.), Diseases of poultry. Iowa State University Press, Ames, Ia. 26. Schlauder, G. G., and I. K. Mushahwar. 2001. Genetic heterogeneity of hepatitis E virus. J. Med. Virol. 65:282-292. 27. Sun, Z. F., F. F. Huang, P. G. Halbur, S. K. Schommer, F. W. Pierson, T. E. Toth, and X. J. Meng. 2003. Use of heteroduplex mobility assays (HMA) for presequencing screening and identification of variant strains of avian and swine hepatitis E viruses. Vet. Microbiol. 96:165-176. 84 28. Sun, Z. F., C. T. Larsen, A. Dunlop, F. F. Huang, F. W. Pierson, T. E. Toth, and X. J. Meng. 2004. Genetic identification of an avian hepatitis E virus (avian HEV) from healthy chicken flocks and characterization of the capsid gene of 14 avian HEV isolates from chickens with hepatitis-splenomegaly syndrome in different geographic regions of the United States. J. Gen. Virol. 85:693-700. 29. Takahashi, M., T. Nishizawa, H. Miyajima, Y. Gotanda, T. Iita, F. Tsuda, and H. Okamoto. 2003. Swine hepatitis E virus strains in Japan form four phylogenetic clusters comparable with those of Japanese isolates of human hepatitis E virus. J. Gen. Virol. 84:851-862. 30. Tei, S., N. Kitajima, K. Takahashi, and S. Mishiro. 2003. Zoonotic transmission of hepatitis E virus from deer to human beings. Lancet 362:371-373. 31. Thomas, D. L., P. O. Yarbough, D. Vlahov, S. A. Tsarev, K. E. Nelson, A. J. Saah, and R. H. Purcell. 1997. Seroreactivity to hepatitis E virus in areas where the disease is not endemic. J. Clin. Microbiol. 35:1244-1247. 32. Tsarev, S. A., T. S. Tsareva, S. U. Emerson, P. O. Yarbough, L. J. Legters, T. Moskal, and R. H. Purcell. 1994. Infectivity titration of a prototype strain of hepatitis E virus in cynomolgus monkeys. J. Med. Virol. 43:135-142. 33. van der Poel, W. H, F., Verschoor, R., van der Heide, M. I., Herrera, A., Vivo, M. Kooreman, and A. M. de Roda Husman. 2001. Hepatitis E virus sequences in swine related to sequences in humans, The Netherlands. Emerg. Infect. Dis. 7:970976. 34. Wang, Y., H. Zhang, R. Ling, H. Li, and T. J. Harrison. 2000. The complete sequence of hepatitis E virus genotype 4 reveals an alternative strategy for translation of open reading frames 2 and 3. J. Gen. Virol. 81:1675-1686. 35. Wang, Y. C., H. Y. Zhang, N. S. Xia, G. Peng, H. Y. Lan, H. Zhuang, Y. H. Zhu, S. W. Li, K. G. Tian, W. J. Gu, J. X. Lin, X. Wu, H. M. Li, and T. J. Harrison. 2002. Prevalence, isolation, and partial sequence analysis of hepatitis E virus from domestic animals in China. J. Med. Virol. 67:516-521. 36. Williams, T. P. E., C. Kasorndorkbua, P. G. Halbur, G. Haqshenas, D. K. Guenette, T. E. Toth, and X. J. Meng. 2001. Evidence of extrahepatic sites of replication of the hepatitis E virus in a swine model. J. Clin. Microbiol. 39:3040- 85 3046. 37. Wu, J. C., C. M. Chen, T. Y. Chiang, W. H. Tsai, W. J. Jeng, I. J. Sheen, C. C. Lin, and X. J. Meng. 2002. Spread of hepatitis E virus among different-aged pigs: two- year survey in Taiwan. J. Med. Virol. 66:488-492. 86 0 1 3 4 6 8 Weeks Post-inoculation (WPI) 2 10 12 Turkey ID 2786 2792 2872 2873 2793 2798 2800 87 and blood samples were collected from this turkey only for the first 8 weeks. inoculated ones, remained seronegative. Prior to the end of the study, one inoculated turkey (#2872) was necropsied at 9 WPI at 4-8 weeks post-inoculation (WPI). Three un-inoculated control turkeys (#2793, #2798, and #2800), housed separately from #2873) turkeys, and one serologically negative contact control (#2786) housed together with the inoculated ones, seroconverted in seven representative turkeys. All turkeys were seronegative at the beginning of the study. Three inoculated (#2792, #2872, and Fig. 4.1. Cross-species infection of 1-week-old turkeys with avian HEV from a chicken: seroconversion to avian HEV antibody 0.0 0.2 0.4 0.6 0.8 1.0 1.2 1.4 1.6 10-6 10-5 10-4 10-3 10-2 Avian HEV stock dilution 0/2 0/4 0/2 0/4 0/2 0/4 0/2 0/4 0/2 0/4 Contact Inocula Contact Inocula Contact Inocula Contact Inocula Contact 0 Inocula Group 0/4 0/2 0/4 0/2 0/4 0/2 0/4 0/2 0/4 0/2 2 88 0/4 0/2 0/4 1/2 0/4 2/2 0/4 2/2 0/4 2/2 4 0/4 0/2 0/4 1/2 0/4 2/2 0/4 2/2 0/4 2/2 6 0/4 0/2 0/4 1/2 0/4 2/2 0/4 2/2 2/4 2/2 8 No. seropositive / no. tested Weeks post-inoculation (WPI) 0/4 0/2 0/4 1/2 0/4 2/2 0/4 2/2 2/4 2/2 10 0/4 0/2 0/4 1/2 0/4 2/2 0/4 2/2 2/4 2/2 12 Table 4.1. Anti-avian HEV antibody seroconversion by infectivity titration of an avian HEV stock in young SPF chickens NA b (1)/2 0(0)/2 a 0(0)/4 0(0)/2 0(0)/4 0(0)/2 0(0)/4 0(0)/2 0(0)/4 0(0)/2 0(0)/4 Inocula Contact Inocula Contact Inocula Contact Inocula Contact Inocula Contact NA(0)/4 NA (0)/2 NA(0)/4 NA(0)/2 NA(0)/4 NA(0)/2 NA(0)/4 NA(2)/2 NA(0)/4 1 0 Group 0(0)/4 0(0)/2 0(0)/4 0(0)/2 0(0)/4 0(2)/2 0(0)/4 2(2)/2 0(0)/4 2(2)/2 2 NA(0)/4 NA(0)/2 NA(0)/4 NA(1)/2 NA(0)/4 NA(1)/2 NA(0)/4 NA(0)/2 NA(0)/4 NA(1)/2 3 0(0)/4 0(0)/2 0(0)/4 0(0)/2 0(0)/4 0(0)/2 0(0)/4 0(0)/2 0(0)/4 1(1)/2 4 0(0)/4 0(0)/2 0(0)/4 0(0)/2 0(0)/4 0(0)/2 0(0)/4 0(0)/2 1(2)/4 1(1)/2 6 No. positive / no. tested Weeks post-inoculation (WPI) 0(0)/4 0(0)/2 0(0)/4 0(0)/2 0(0)/4 0(0)/2 0(0)/4 0(0)/2 2(1)/4 1(1)/2 8 0(0)/4 0(0)/2 0(0)/4 0(0)/2 0(0)/4 0(0)/2 0(0)/4 0(0)/2 1(0)/4 1(1)/2 10 b 89 No. positive for viremia (or fecal virus shedding in parentheses) / no. tested NA, serum samples not available at this time point c Chickens necropsied at 12 WPI, bile sample was also positive for one chicken (#5357) in the 10-2 dilution group a 10-6 10-5 10-4 10-3 10-2 dilution Avian HEV stock 0(0)/4 0(0)/2 0(0)/4 0(0)/2 0(0)/4 0(0)/2 0(0)/4 0(0)/2 0(0)/4 1(1)/2 c 12 Table 4.2. Viremia and fecal virus shedding of avian HEV in young chickens experimentally inoculated with different doses of an avian HEV stock Contact control Avian HEV Inocula -a 2873 2875 - - 2872 2786 - 2792 - 2790 - -a 2788 2791 - 0 2776 ID No. - - - - - - - 1 - - - - - -b -b 2 - - - - - 3 - - + - - 4 II None 90 9 turkeys (2787, 2789, 2793, 2795-2800) a Two turkeys died of anaphylaxis within 48 hours post-inoculation; -, negative; +, positive b Two turkeys died during routine blood collection process at 2 WPI c One turkey necropsied at 9 WPI d One turkey necropsied at 11 WPI I Group - - + + - - 6 Weeks post-inoculation (WPI) - + + + + + 8 - + + c + + 10 - + + + d 12 Table 4.3. Anti-avian HEV antibody seroconversion in turkeys experimentally inoculated with avian HEV from a chicken Avian HEV I b a 2786 -/- -/- -/- -/- 2873 -/- -/- -/- 2872 2875 -/- -/- 2792 b -/- -/- -/- 1 -/- -/-/- -/- 0 2791 2790 2788 2776 ID No. c c -/- -/- +/- -/- -/- -/- -/- 2 -/- -/- +/- -/- -/- 3 -/- -/- -/+ -/- +/- 4 II None 91 NA/- NA/- NA/+ NA/- d NA /NA/- 5 -/- -/- +/+ -/- -/+ +/- 6 Weeks post-inoculation (WPI) 9 turkeys -/-/-/-/-/(2787, 2789, 2793, 2795-2800) a Viremia / fecal virus shedding; -, negative; +, positive b Two turkeys died of anaphylaxis within 48 hours post-inoculation c Two turkeys died during routine blood collection process at 2 WPI d NA, serum sample not available at this time point e One turkey necropsied at 9 WPI f One turkey necropsied at 11 WPI, bile sample was also positive for this turkey Contact control Inocula Group NA/- NA/- NA/- NA/- NA/- NA/+ 7 -/- -/- -/- -/- -/- -/- 8 -/- -/- -/- e -/- -/- 10 Table 4.4. Detection of avian HEV RNA in serum and fecal samples of turkeys experimentally inoculated with avian HEV from a chicken -/- -/- -/- -/- f 12 CHAPTER V Characterization of the ORF3 Proteins of Human, Swine and Avian Hepatitis E Viruses (HEV): Identification of Antigenic Cross-Reactivity between Swine HEV and Human HEV but Failure to Detect the ORF3 Protein in Native Virions Z. F. Sun, and Others (Co-authors To Be Determined) Journal of Virology (To Be Submitted). 5.1 Abstract Hepatitis E is a major public health problem in many developing countries and is also endemic in some industrialized nations. Hepatitis E virus (HEV) is a non-enveloped RNA virus, which contains three open reading frames (ORF). Little is known regarding the characteristics of the small ORF3 protein, largely due to the fact that no reliable cell culture system is available for HEV. In this study, ORF3 proteins of avian and swine HEVs were expressed in Escherchia coli, and purified by BugBuster His-Bind Purification System. Western blot analysis showed that avian HEV ORF3 protein is unique and does not share common antigenic epitopes with those of swine and human HEVs. However, swine HEV (genotype 3) and human HEV (genotype 1) ORF3 proteins cross-react with each other antigenically. To determine the virion proteins of HEV, infectious stocks of avian and swine HEVs were produced in SPF chickens and pigs, respectively, and virions were subsequently purified by sucrose density gradient centrifugation. Virion proteins were characterized from the purified native virions by SDS-PAGE and Western blot analysis. Two major forms of ORF2 proteins of avian HEV were identified: a 56 kDa and a 80 kDa proteins. Multiple immunoreactive forms of ORF2 proteins of swine HEV were also observed: 40 kDa, 53 kDa, 56 kDa and 72 kDa. However, the ORF3 protein was not detected from the native virions of avian HEV or swine HEV. These findings provide direct evidence that ORF2 indeed encodes 92 a structural protein whereas ORF3 does not. This is the first report on the analyses of viral proteins of avian and swine HEVs using purified virions. 93 5.2 Introduction Hepatitis E is an acute disease that is transmitted by the fecal-oral route, usually through contaminated water (1, 7, 34, 35). The overall fatality rate is about 1% in young adults, however it can reach up to 15-25% in pregnant women (7, 12, 18, 34). The severity of illness increases with age. The disease is a major public health concern in many developing countries in Asia, Africa and South America where the hygiene condition is poor (2, 16, 51, 52). Sporadic cases of hepatitis E also occur in some industrialized countriess, including the United States (5, 9, 15, 23, 29, 33, 39, 40, 57). The causative agent of this disease, hepatitis E virus (HEV), is a positive sense, single-stranded non-enveloped RNA virus of about 27-34 nm in diameter. The genome of HEV is about 7.2 kb and contains three open reading frames (ORFs). It is predicated that ORF1 encodes putative nonstructural proteins, ORF2 encodes a putative major capsid protein, and ORF3 encodes a small protein of unknown function (1, 7, 34). HEV belongs to the genus Hepevirus, family Hepeviridae (8). Due to the lack of an efficient cell culture system, many aspects of HEV biology are still not known (7). The existing information about HEV is mainly obtained from recombinant technologies and no data are available from purified native virions so far since HEV can not be propagated (19, 38, 47). It is still controversal regarding the properties of HEV structural proteins, especially whether or not ORF3 protein is a structural component of the native virions (48, 49, 56). The identification and characterization of the animal strains of HEV from pigs and chickens afforded us opportunities to study HEV replication and pathogenesis in chicken and pig models (3, 4, 5, 6, 10, 13, 15, 24, 28, 31, 36, 37, 41, 44, 50, 53, 54, 55). Using the swine and chicken animal models, sufficient amounts of infectious viruses can be generated for the characterization of virons. It has been demonstrated that swine HEV can cross species barriers and infect nonhuman primates and an U.S. strain of human HEV can infect pigs (11, 25, 26, 28, 46). Swine HEV isolates were found to be closely related to and in some cases, indistinguishable from, isolates of human HEV (15, 24, 29, 44, 45, 50, 53). It has been shown that swine veterinarians and pig handlers are at higher risk of zoonotic HEV infection than normal blood donors (22, 27). However, a relatively high prevalence of anti-HEV was also found in normal U.S. blood donors, suggesting that pigs are not the only source of infection and that multiple sources of exposure to HEV may exist (27). We previously showed that avian HEV also can 94 cross species barriers and infect turkeys (42), although avian HEV failed to infect 2 rhesus monkeys (17). There is no report as to whether or not avian HEV infects humans. Like swine HEV, avian HEV is also genetically related to human HEV and its ORF2 protein shares common antigenic epitope with human and swine HEVs (13, 14, 17, 41). The discovery of avian HEV and its close antigenic and genetic relatedness to human HEV warrants further investigation on the potential risk of avian HEV infection in humans. Since we showed that the ORF2 protein of avian HEV cross-reacts antigenically with human and swine HEVs (14), it is therefore important to characterize the small ORF3 protein for its antigenic relatedness with human and swine HEVs. It is also important to know whether or not the ORF3 protein is a structural component of virions. 95 5.3 Materials and Methods 5.3.1 Cloning and Expression of the Truncated Avian HEV ORF2 Capsid Protein The truncated avian HEV ORF2 capsid protein was expressed in E. coli strain BL21(DE3)pLysS (14) and purified with the BugBuster His-Bind purification kit (Novagen), based on the affinity of His-Bind resin for His-tagged fusion protein (41, 43). The expressed protein was confirmed with monoclonal antibodies to the His tag and Xpress epitope, and with a polyclonal antiserum against avian HEV by Western blot analyses. 5.3.2 Expression and Purification of ORF3 Proteins of Avian HEV and Swine HEV The full length ORF3 genes of avian and swine HEVs were cloned, expressed and characterized in E. coli (Figs. 5.1 and 5.2). Briefly, the 264 bp ORF3 gene of avian HEV was amplified by RT-PCR (Fig. 5.3) with a set of primers (forward primer 5'CCCGGATCCCCATGTGCCTTAGCTGCCAGT-3' and reverse primer 5'CCCGAATTCCTACATCTGGTACCGTGCGAG-3'). To facilitate the subsequent cloning steps, a BamH I site and an EcoR I site (underlined) were introduced at the 5' ends of the sense and antisense primers, respectively. Platinum PCR SuperMix High Fidelity (Invitrogen) was used for the PCR amplification. The amplified ORF3 gene of avian HEV was cloned in frame with the sequences coding for His tag and Xpress epitope located upstream of the multiple cloning site of the pRSET-C expression vector (Invitrogen) (Fig. 5.1). The recombinant plasmids were transformed into BL21 Star(DE3)pLysS One Shot chemically competent cells (Invitrogen, Carlsbad, CA) (41, 43), which produced T7 polymerase, and the expression of the fusion avian HEV ORF3 protein was driven by the addition of 1 mM IPTG. The ORF3 protein of avian HEV was purified with the BugBuster His-Bind purification kit (Novagen), based on the affinity of His-Bind resin for the Histagged fusion protein, and confirmed with monoclonal antibodies against the fused His tag and Xpress epitope (Invitrogen), as well as with the polyclonal antiserum against avian HEV by Western blot. For swine HEV ORF3, similar strategies were utilized for its cloning, 96 expression and purification. The 369 bp ORF3 gene of swine HEV was amplified by RTPCR (Fig. 5.4) with a set of primers (forward primer 5'CCCGGATCCGAATGAATAACATGTCTTTTG-3' and reverse primer 5'CCCGAATTCTCAGCGGCGCAGCCCCAGCTG-3'). A BamH I site and an EcoR I site (underlined) were introduced at the 5' ends of the sense and antisense primers, respectively to facilitate the subsequent cloning steps. 5.3.3 Generation of Antisera against the ORF3 Proteins of Avian, Swine and Human HEVs Mono-specific antisera against avian, swine and human HEV ORF3 proteins were generated by immunizing Balb/c mice with respective recombinant protein. A total of nine 4week-old female Balb/c mice were purchased (Charles River Laboratories, Wilmington, MA) and divided into three groups of 3 each. Mice in each group were immunized intramuscularly with 25 µg of recombinant ORF3 proteins of avian, swine or human HEVs (BioDesign, Inc., Saco, ME) in Freund’s Adjuvant Incomplete (FAI) (Sigma), respectively. Three booster injections with aqueous protein (without FAI) were given at two-week intervals. Sera were collected prior to immunization and at seven weeks after the initial immunization. Antibodies to each antigen were titrated by ELISA. At nine weeks after initial immunization, blood was collected under anesthesia and the mice were then necropsied. Convalescent sera against swine HEV and avian HEV were obtained from SPF pigs and chickens experimentally infected with the respective strains of HEVs (24, 42). The antiserum against the ORF2 capsid protein of human HEV (strain Sar-55) had been generated previously by immunizing SPF pigs with the baculovirus-expressed HEV Sar-55 capsid protein (24, 38). The rabbit antiserum to human HEV ORF3 peptide and the rhesus macaque convalescent serum against human HEV ORF3 protein used in this study were gifts from Dr. Suzanne Emerson (National Institutes of Health, Bethesda, Maryland, USA). The rabbit mono-specific antiserum against ORF2 peptide of avian HEV was kindly provided by Dr. Eric Zhou (Iowa State University, Ames, Iowa, USA). 97 5.3.4 Antigenic Cross-Reactivity among the ORF3 Proteins of Avian, Swine and Human HEVs We previously showed that the ORF2 protein of avian HEV cross-reacted with swine and human HEVs (14). Therefore, Western blot analysis was used to determine if the ORF3 protein of avian HEV shares antigenic epitopes with those of swine and human HEVs. The purified recombinant ORF3 proteins of avian, swine and human HEVs were boiled for 5 min. The denatured proteins were separated using 15% polyacrylamide gel (1 µg/lane) and transferred onto a nitrocellulose membrane (Schleicher & Schuell). After blocking in Trisbuffered saline (20 mM Tris-HCl, pH 7.4, 500 mM NaCl) (TBS) containing 1.5% BSA (FisherBiotech) and 1.5% natural nonfat dry milk (Carnation) at room temperature for 1 h, the membrane was cut into separate strips. The strips were then incubated at room temperature for 1 h with a 1:100 dilution of antiserum against the ORF3 protein of either avian, swine or human HEV in TBS containing 0.05% Tween 20 (TBST). The strips were washed twice with TBST and twice with TBS. Following 1 h incubation with HRPconjugated goat anti-mouse IgG (1:1000, KPL) or HRP-conjugated goat anti-rabbit IgG (1:1000, KPL), the strips were washed again as described above and the immunocomplexes were detected using 4-chloro-1-naphthol (Sigma) (14, 43). 5.3.5 Purification of Avian and Swine HEV Virions Avian and swine HEV virions were purified by sucrose density gradient centrifugation from positive fecal and bile materials of experimentally infected chickens and pigs, respectively (20, 30, 32, 42). Briefly, positive fecal and bile materials were resuspended in cold PBS (1:10 w/v). The suspension was clarified by centrifugation for 10 minutes at 4 °C at 3,000 rpm (Sorvall GSA rotor). The virus-containing supernatant was gently overlaid on top of 0.5 ml 20% glycerol, and viruses were pelleted by centrifugation for 4 hours at 4 °C at 28,000rpm (SW28 rotor). The pellets were resuspended in 1 ml 1X PBS. About 1 ml of viron suspension was gently loaded on top of a step sucrose gradient (2 ml 30%/2 ml 45%) and virions were banded by centrifugation for 8 hours at 4°C at 45,000 rpm (SW55 Ti rotor). A band was visualized at the sucrose interface. A sterile needle was punctured into the wall of the ultra-centrifuge tube and the virus layer was transferred to a fresh 5 ml ultra-centrifuge 98 tube. The virus layer was mixed with 3 ml of cold PBS and then gently overlaid on top of 0.5 ml of 20% glycerol. The virons were pelleted by centrifugation for 4 hours at 4°C at 45,000 rpm (SW55 Ti rotor). Each virion pellet was resuspended in 0.5 ml of 1X PBS and stored at 80°C until use. 5.3.6 Characterization of Viron Proteins of Avian HEV and Swine HEV Viron proteins of avian and swine HEVs were analyzed using SDS-PAGE and Western blot (19, 20, 30, 32, 38). Briefly, purified virions were denatured for 5 minutes at 100°C in the presence of 60 mM Tris, pH 6.8, 2% sodium dodecyl sulphate (SDS), 2% βmercaptoethanol, and 5% glycerol, and subsequently separated on 10-20% gradient polyacrylamide gels (Bio-Rad). Proteins were visualized by staining gels with Coomassie Brilliant blue (Sigma). After transferring the separated proteins onto nitrocellulose membranes by electroblot techniques, the membranes were blocked at room temperature for 1 h and cut into separate strips. The strips were incubated with convalescent sera (1:100) against avian HEV and swine HEV, as well as with the antisera against avian HEV ORF2 or human HEV ORF2 proteins, or with the antisera against avian HEV ORF3, swine HEV ORF3 or human HEV ORF3 proteins, followed by incubation with 1:1000 diluted secondary antibodies (HRP-conjugated goat anti-chicken IgG, HRP-conjugated goat anti-swine IgG, HRP-conjugated goat anti-rabbit IgG or HRP-conjugated goat anti-mouse IgG). The chromogenic substrate – 4-chloro-1-naphthol (Sigma) was used to detect immunoreactive proteins. 5.3.7 ELISA to Detect Antibodies against Avian HEV ORF3 Protein in Chickens Experimentally Infected with Avian HEV The purified avian HEV ORF3 protein (0.05 µg per well) was used as the antigen to standardize an ELISA to detect antibodies against avian HEV ORF3 protein in experimentally infected chickens collected from a previous study (42). Chicken sera were diluted at 1:100 and added to ELISA plates (100 µl/well). A horseradish peroxidase (HRP)conjugated rabbit anti-chicken IgG (Sigma) was used as the secondary antibody. The cutoff value of OD405 for this assay was 0.188, which was the mean value of thirty-six pre99 inoculation chicken sera plus 3 times of standard deviation (SD). Sera from serologically negative SPF chickens were used as negative controls, and convalescent sera from SPF chickens experimentally infected with avian HEV were included as positive controls. All serum samples were tested at least twice. 5.4 Results 5.4.1 Expression and Purification of the Truncated ORF2 Capsid Protein of Avian HEV The truncated ORF2 capsid protein of avian HEV was expressed in E. coli strain BL21(DE3)pLysS and induced with IPTG. The fusion protein was observed on the SDSPAGE at the expected size of approximately 32 kDa and the maximum expression occurred at 3-6 h post-induction with IPTG. As previously reported (14), the recombinant fusion protein was mainly expressed in insoluble form. About 6.951 mg of purified protein was obtained from 500 ml of bacterial cultures, and used in the study. 5.4.2 Expression and Purification of ORF3 Proteins of Avian HEV and Swine HEV The ORF3 proteins of avian and swine HEVs were expressed in bacterial cells with high yields upon induction with IPTG, and were observed on the SDS-PAGE at the expected sizes of approximately 12 kDa and 15 kDa, respectively. Samples collected at different time points indicated that the maximum expression occurred at approximately 5-6 h and 7-12 h after IPTG induction, respectively. Western blot analyses using a monoclonal antibody against the Xpress epitope or His tag confirmed the expressions (Figs. 5.5A and 5.5B). Like the recombinant ORF2 capsid protein of avian HEV, the ORF3 fusion proteins of avian HEV and swine HEV were also mainly expressed as an insoluable form (Figs. 5.5A and 5.5B). About 9.906 mg and 5.862 mg of purified ORF3 proteins of avian HEV and swine HEV were obtained from 500 ml of bacterial cultures, respectively. The expressed ORF3 proteins of avian HEV and swine HEV were also observed in dimer form (Figs. 5.5A and 5.5C). 100 5.4.3 Titration of Antisera against the ORF3 Proteins of Avian, Swine and Human HEVs Mono-specific antisera against ORF3 proteins of avian, swine and human HEVs were generated in Balb/c mice. The titers of the antibodies were determined by ELISA and the ELISA titers reached up to 105 -106 after three booster doses of immunizations. 5.4.4 Antigenic Cross-Reactivity among the ORF3 Proteins of Avian, Swine and Human HEVs by Western Blot Analyses As expected, recombinant ORF3 fusion proteins of avian and swine HEVs reacted well with HRP-conjugated anti-His G antibody as well as with mono-specific mouse antisera against the ORF3 proteins of avian HEV or swine HEV, respectively (Fig. 5.6A). The purified recombinant ORF3 proteins of avian HEV or swine HEV also reacted well with the convalescent sera from SPF chickens or pigs experimentally infected with avian HEV or swine HEV, respectively (Fig. 5.6A). Human HEV ORF3 protein reacted with mono-specific mouse antisera against human HEV ORF3 and swine HEV ORF3 proteins, as well as with swine HEV convalescent serum (Fig. 5.6B). However, avian HEV ORF3 protein did not react with monospecific mouse anti-swine HEV ORF3 or mouse anti-human HEV ORF3 antisera. Conversely, swine HEV ORF3 and human HEV ORF3 proteins did not react with avian HEV convalescent serum or with mouse anti-avian HEV ORF3 serum (Figs. 5.6A and 5.6B). 5.4.5 Purification and Characterization of Virion Proteins of Avian HEV and Swine HEV Avian and swine HEV virions were purified by sucrose density gradient centrifugation. Three major forms of ORF2 proteins of avian HEV (56 kDa, 80 kDa and 130 kDa) were identified by Western blot analysis (Fig. 5.7). Multiple forms of immunoreactive ORF2 proteins of swine HEV were identified, and the major proteins were 40 kDa, 53 kDa, 56 kDa, and 72 kDa (Fig. 5.8). Two smaller sizes of ORF2 proteins (approximately 18 kDa and 28 kDa) were also observed in the native virions of swine HEV when reacted with anti-human HEV 101 ORF2 antibody (data not shown). However, ORF3 protein was not detected from the purified native virions of avian HEV or swine HEV (Figs. 5.7 and 5.8). 5.4.6 Detection of Antibodies against Avian HEV ORF3 Protein in Chickens Experimentally Infected with Avian HEV Unlike the antibodies against avian HEV ORF2 protein, antibodies against avian HEV ORF3 protein were detected in only a few infected chickens with relative low OD405 values of 0.198-0.655 (Table 5.1). In some chickens (#5357 and #5354) with high levels of anti-avian HEV ORF2 antibodies (1.474-1.560 OD405 values), anti-avian HEV ORF3 antibodies (0.0740.167 OD405 value) were undetectable. However, anti-avian HEV ORF3 antibodies were detected from chicken #5352 that was negative for avian HEV ORF2 antibody. In general, the detection of anti-avian HEV ORF3 antibodies in experimentally infected chickens was inconsistent, and at relative low level compared to anti-avian HEV ORF2 antibodies. 5.5 Discussion We have previously reported that the putative ORF2 capsid protein of avian HEV shared common antigenic epitopes with those of swine and human HEVs as well as with the Australian chicken big liver and spleen disease virus (BLSV) (14). However, the antigenic cross-reactivity, if any, among the ORF3 proteins of avian, swine and human HEVs is not known. It is also not known if avian HEV can infect humans and, if so, how prevalent the avian HEV antibody is in human populations. Since cross-reactivity among the ORF2 capsid proteins of avian, swine and human HEVs exists, it is not appropriate to use the avian HEV ORF2 protein as the antigen for assessing the zoonotic risk of potential avian HEV infection in humans. In this study, we report that the avian HEV ORF3 protein is unique and has no antigenic cross-reactivity with the antisera against the swine HEV and human HEV ORF3 proteins. This unique antigenic feature of avian HEV ORF3 is supported by the fact that avian HEV only shares 29-34% nucleotide sequence identity in ORF3 gene with that of human and swine HEVs (17). We also found in this study that the ORF3 protein of avian HEV is less immunogenic and with low affinity compared to the ORF2 capsid protein when tested in experimentally infected chickens by ELISA and Western blot analysis. Even in chickens with 102 high titers of anti-avian HEV ORF2 antibodies, anti-avian HEV ORF3 antibodies were hardly detectable. Similar results were also observed in Western blot analysis (data not shown). These data strongly suggest that the ORF3 protein of avian HEV is poorly immunogenic and may not be a structural protein. Due to the lack of a cell culture system, the biology of HEV is largely unknown (7, 19). So far, knowledge regarding the characteristics of HEV proteins were solely based on the recombinant proteins expressed in baculovirus, and bacteria (19, 38, 47). The properties of viral proteins in the native virions are not known. The ORF3 protein is believed to be phosphorylated and unglycosylated (48, 56). It is not clear whether the ORF3 protein is a nonstructural protein or a component of virions (49). The discoveries of animal strains of HEVs (13, 24, 41) make it possible to characterize HEV virion proteins. In this study, avian and swine HEV virions were purified by sucrose density gradient centrifugation, and viral proteins were characterizeded by SDS-PAGE and Western blot analysis. Three major forms of ORF2 proteins of avian HEV with sizes of about 56 kDa, 80 kDa and 130 kDa were identified. Similarly, multiple immunoreactive forms of ORF2 proteins of swine HEV (40 kDa, 53 kDa, 56 kDa and 72 kDa) were also detected in native virions of swine HEV. Additionally, immunoreactive proteins with other molecular weights but less abundancy were also observed. These results suggest that a stochastic cascade of proteolytic cleavages occurs during the infection to render capsid proteins of various sizes, which is in agreement with the reports that post-translational proteolytic cleavage, which is necessary for particle formation and proteolytic processing of ORF2, takes place before or during HEV capsid assembly (19, 21, 49). The product of 130 kDa could be the result of a noncovalent homodimeric complex of the mature form of the ORF2 protein (47). It has been shown that the ORF2 protein can selfassociate in the absence of ORF3 to form dimers and virus-like particles (VLPs) (49). It has been reported that the HEV ORF2 protein is synthesized as a large precursor of about 82 kDa, which is subsequently processed into a 74 kDa mature protein by cleavage of the signal sequence and glycosylated into an 88 kDa protein (19). Based on our observation of different sizes of ORF2 proteins and the lack of detection of ORF3 protein from the purified virions of both avian and swine HEVs, we conclude that ORF2 indeed encodes a structural protein of HEV. However, our data showed that the ORF3 protein of avian and swine HEVs is apparently not a component of HEV virions as anti-avian 103 HEV ORF3 antibodies could not be consistently detected in experimentally infected chickens and ORF3 protein is undetectable in purified virions. 5.6 Acknowledgements We thank Dr. Suzanne Emerson (National Institutes of Health, Bethesda, Maryland, USA) for providing the mono-specific rabbit anti-HEV ORF3 peptide serum and rhesus macaque convalescent serum against ORF3 protein of human HEV; and Dr. Eric Zhou (Iowa State University, Ames, Iowa, USA) for providing the mono-specific rabbit antiserum against ORF2 peptide of avian HEV. We are grateful to Denis Guenette for his technical assistance. This work is supported by grants from the NIH (AI 01653, AI 46505 and AI 50611) and from the USDA (NRI 35204-12531). 104 5.7 References 1. Aggarwal, R., and K. Krawczynski. 2000. Hepatitis E: an overview and recent advances in clinical and laboratory research. J. Gastroenterol. Hepatol. 15:9-20. 2. Arankalle, V. A., M. S. Chadha, S. A. Tsarev, S. U. Emerson, A. R. Risbud, K. Banerjee, and R. H. Purcell. 1994. Seroepidemiology of water-borne hepatitis in India and evidence for a third enterically-transmitted hepatitis agent. Proc. Natl. Acad. Sci. USA 91:3428-3432. 3. Billam, P., F. F. Huang, Z. F. Sun, D. K. Guenette, F. W. Pierson, R. B. Duncan, T. E. Toth, and X. J. Meng. 2004. Systematic pathogenesis and replication of a strain of the hepatitis E virus (HEV) in its natural host: avian HEV infections in specific-pathogen-free adult chickens. J. Virol. (In press). 4. Chandler, J. D., M. A. Riddell, F. Li, R. J. Love, and D. A. Anderson. 1999. Serological evidence for swine hepatitis E virus infection in Australian pig herds. Vet. Microbiol. 68:95105. 5. Choi, I.-S., H.-J. Kwon, N.-R. Shin, and H. S. Yoo. 2003. Identification of swine hepatitis E virus (HEV) and prevalence of anti-HEV antibodies in swine and human populations in Korea. J. Clin. Microbiol. 41:3602-3608. 6. Clayson, E. T., K. S. Innis, S. Myint, S. Narupiti, D. W. Vaughn, S. Giri, P. Ranabhat, and M. P. Shrestha. 1995. Detection of hepatitis E virus infections among domestic swine in the Kathmandu Valley of Nepal. Am. J. Trop. Med. Hyg. 53:228- 232. 7. Emerson, S. U., and R. H. Purcell. 2003. Hepatitis E virus. Rev. Med. Virol. 13:145-154. 8. Emerson, S. U., D. Anderson, A. Arankalle, X. -J. Meng, M. Purdy, G. G. Schlauder, and S. A. Tsarev. 2004. Hepevirus, p. 851-855. In C.M. Fauquet, M. A. Mayo, J. Maniloff, U. Desselberger, and L.A. Ball. (ed.), Virus Taxonomy, VIIIth Report of the ICTV, Elsevier/Academic Press, London. 9. Erker, J. C., S. M. Desai, G. G. Schlauder, G. J. Dawson, and I. K. Mushahwar. 1999. A hepatitis E virus variant from the United States: molecular characterization and transmission in cynomolgus macaques. J. Gen. Virol. 80:681-690. 10. Garkavenko, O., A. Obriadina, J. Meng, D. A. Anderson, H. J. Benard, B. A. Schroeder, Y. E. Khudaakov, H. A. Fields, and M . C. Croxson. 2001. Detection and characterization of swine hepatitis E virus in New Zealand. J. Med. Virol. 65:525-529. 105 11. Halbur, P. G., C. Kasorndorkbua, C. Gilbert, D. Guenette, M. B. Potters, R. H. Purcell, S. U. Emerson, T. E. Toth, and X. J. Meng. 2001. Comparative pathogenesis of infection of pigs with hepatitis E viruses recovered from a pig and a human. J. Clin. Microbiol. 39:918-923. 12. Hamid, S. S., S. M. Washim Jafri, H. Khan, H. Shah, Z. Abbas, and H. Fields. 1996. Fulminant hepatic failure in pregnant women: acute fatty liver or acute viral hepatitis? J. Hepatol. 25:20-27. 13. Haqshenas, G., H. L. Shivaprasad, P. R. Woolcock, D. H. Read, and X. J. Meng. 2001. Genetic identification and characterization of a novel virus related to human hepatitis E virus from chickens with hepatitis-splenomegaly syndrome in the United States. J. Gen. Virol. 82:2449-2462. 14. Haqshenas, G., F. F. Huang, M. Fenaux, D. K. Guenette, F. W. Pierson, C. T. Larsen, H. L. Shivaprasad, T. E. Toth, and X. J. Meng. 2002. The putative capsid protein of the newly identified avian hepatitis E virus shares antigenic epitopes with that of swine and human hepatitis E viruses and chicken big liver and spleen disease virus. J. Gen. Virol. 83:2201-2209. 15. Hsieh, S. Y., X. J. Meng, Y. H. Wu, S. T. Liu, A. W. Tam, D. Y. Lin, and Y. F. Liaw. 1999. Identity of a novel swine hepatitis E virus in Taiwan forming a monophyletic group with Taiwan isolates of human hepatitis E virus. J. Clin. Microbiol. 37:3828-3834. 16. Huang, C. C., D. Nguyen, J. Fernandez, K. Y. Yun, K. E. Fry, D. W. Bradley, A. W. Tam, and G. R. Reyes. 1992. Molecular cloning and sequencing of the Mexico isolate of hepatitis E virus (HEV). Virology 191:550-558. 17. Huang, F. F., Z. F. Sun, S. U. Emerson, R. H. Purcell, H. L. Shivaprasad, F. W. Pierson, T. E. Toth and X. J. Meng. 2004. Determination and analysis of the complete genomic sequence of avian hepatitis E virus (avian HEV) and attempts to infect rhesus monkeys with avian HEV. J. Gen. Virol. 85:1609-1618. 18. Hussaini, S. H., S. J. Skidmore, P. Richardson, L. M. Sherratt, B. T. Cooper, and J. G. O’Grady. 1997. Severe hepatitis E infection during pregnancy. J. Viral. Hepat. 4:5154. 19. Jameel, S., M. Zafrullah, . H. Ozdener, and S. K. Panda. 1996. Expression in animal cells and characterization of the hepatitis E virus structural proteins. J. Virol. 70:207-216. 106 20. Le Gall, G., E. Boilletot, and J. P. Morisse. 1992. Viral haemorragic disease of rabbit: purification and characterization of a strain isolated in France. Ann. Rech. Vet. 23:381-387. 21. Li, T. C., Y. Yamakawa, K. Suzuki, M. Tatsumi, M. A. Razak, T. Uchida, N. Takeda, T. Miyamura. 1997. Expression and self-assembly of empty virus-like particles of hepatitis E virus. J. Virol. 71, 7207-7213. 22. Mast, E. E., I. K. Kuramoto, M. O. Favorov, V. R. Schoening, B. T. Burkholder, C. N. Shapiro, and P. V. Holland. 1997. Prevalence of and risk factors for antibody to hepatitis E virus seroreactivity among blood donors in Northern California. J. Infec. Dis. 176:34-40. 23. McCrudden, R., S. O’Connell, T. Farrant, S. Beaton, J. P. Iredale, and D. Fine. 2000. Sporadic acute hepatitis E in the United Kingdom: an underdiagnosed phenomenon? Gut 46:732-733. 24. Meng, X. J., R. H. Purcell, P. G. Halbur, J. R. Lehman, D. M. Webb, T. S. Tsareva, J. S. Haynes, B. J. Thacker, and S. U. Emerson. 1997. A novel virus in swine is closely related to the human hepatitis E virus. Proc. Natl. Acad. Sci. USA 94:9860-9865. 25. Meng, X. J., P. G. Halbur, M. S. Shapiro, S. Govindarajan, J. D. Bruna, I. K. Mushahwar, R. H. Purcell, and S. U. Emerson. 1998. Genetic and experimental evidence for cross-species infection by the swine hepatitis E virus. J. Virol. 72:9714-9721. 26. Meng, X. J. 2000. Novel strains of hepatitis E virus identified from humans and other animal species: is hepatitis E a zoonosis? J. Hepatol. 33:842-845. 27. Meng, X. J., B. Wiseman, F. Elvinger, D. K. Guenette, T. E. Toth, R. E. Engle, S. U. Emerson, and R. H. Purcell. 2002. Prevalence of antibodies to the hepatitis E virus in veterinarians working with swine and in normal blood donors in the United States and other countries. J. Clin. Microbiol. 40:117-22. 28. Meng, X. J. 2003. Swine hepatitis E virus: cross-species infection and risk in xenotransplantation. Curr. Topics Microbiol. Immunol. 278:185-216. 29. Nishizawa, T., M. Takahashi, H. Mizuo, H. Miyajima, Y. Gotanda, and H. Okamoto. 2003. Characterization of Japanese swine and human hepatitis E virus isolates of genotype IV with 99% identity over the entire genome. J. Gen. Virol. 84:1245-1251. 107 30. Ohlinger, V. F., B. Haas, G. Meyers, F. Weiland, and H. J. Thiel. 1990. Identification and characterization of the virus causing rabbit hemorrhagic disease. J. Virol. 64:33313336. 31. Okamoto, H., M. Takahashi, T. Nishizawa, K. Fukai, U. Muramatsu, and A. Yoshikawa. 2001. Analysis of the complete genome of indigenous swine hepatitis E virus isolated in Japan. Biochem. Biophys. Res. Commun. 289:929-936. 32. Parra, F., and M. Prieto. 1990. Purification and characterization of a calicivius as the causative agent of a lethal hemorrhagic disease in rabbits. J. Virol. 64:4013-4015. 33. Pina, S., M. Buti, M. Cotrina, J. Piella, and R. Girones. 2000. HEV identified in serum from humans with acute hepatitis and in sewage of animal origin in Spain. J. Hepatol. 33:826-833. 34. Purcell, R. H. 1996. Hepatitis E virus, p. 2831-2843. In B. N. Fields, D. M. Knipe, and P. M. Howley, et al. (ed.), Fields virology, 3rd ed., vol 2. Lippincott-Raven Publishers, Philadelphia, Pa. 35. Reyes, G. R. 1997. Overview of the epidemiology and biology of the hepatitis E virus, p. 239-258. In R. A. Willson (ed.), Viral hepatitis. Marcel Dekker, Inc., New York, N. Y. 36. Riddell, C. 1997. Hepatitis-splenomegaly syndrome, p. 1041. In B. W. Calnek, H. J. Barnes, C. W. Beard, et al. (ed.), Diseases of poultry. Iowa State University Press, Ames, Ia. 37. Ritchie, S. J., and C. Riddell. 1991. “Hepatitis-splenomegaly” syndrome in commercial egg laying hens. Can. Vet. J. 32:500-501. 38. Robinson, R. A., W. H. Burgess, S. U. Emerson, R. S. Leibowitz, S. A. Sosnovtseva, S. Tsarev, and R. H. Purcell. 1998. Structural characterization of recombinant hepatitis E virus ORF2 proteins in baculovirus-infected insect cells. Protein Expr. Purif. 12:75-84. 39. Schlauder, G. G., G. J. Dawson, J. C. Erker, P. Y. Kwo, M. F. Knigge, D. L., Smalley, J. E. Rosenblatt, S. M. Desai, and I. K. Mushahwar. 1998. The sequence and phylogenetic analysis of a novel hepatitis E virus isolated from a patient with acute hepatitis reported in the United States. J. Gen. Virol. 79:447-456. 40. Schlauder, G. G., S. M. Desai, A. R. Zanetti, N. C. Tassopoulos, and I. K. Mushahwar. 1999. Novel hepatitis E virus (HEV) isolates from Europe: evidence for additional genotypes of HEV. J. Med. Virol. 57:243-251. 108 41. Sun, Z. F., C. T. Larsen, A. Dunlop, F. F. Huang, F. W. Pierson, T. E. Toth, and X. J. Meng. 2004. Genetic identification of an avian hepatitis E virus (avian HEV) from healthy chicken flocks and characterization of the capsid gene of 14 avian HEV isolates from chickens with hepatitis-splenomegaly syndrome in different geographic regions of the United States. J. Gen. Virol. 85:693-700. 42. Sun, Z. F., C. T. Larsen, F. F. Huang, P. Billam, F. W. Pierson, T. E. Toth, and X. J. Meng. 2004. Generation and infectivity titration of an infectious stock of avian hepatitis E virus (HEV) in chickens and cross-species infection of turkeys with avian HEV. J. Clin. Microbiol. 42:2658-2662. 43. Sun, Z. F., and X. J. Meng. 2004. Antigenic cross-reactivity between the nucleocapsid protein of severe acute respiratory syndrome (SARS) coronavirus and polyclonal antisera of antigenic group I animal coronaviruses: implication for SARS diagnosis. J. Clin. Microbiol. 42:2351-2352. 44. Takahashi, M., T. Nishizawa, and H. Okamoto. 2003. Identification of a genotype III swine hepatitis E virus that was isolated from a Japanese pig born in 1990 and that is most closely related to Japanese isolates of human hepatitis E virus. J. Clin. Microbiol. 41:13421343. 45. Tei, S., N. Kitajima, K. Takahashi, and S. Mishiro, 2003. Zoonotic transmission of hepatitis E virus from deer to human beings. Lancet 362:371-373. 46. Tien, N. T., H. T. Clayson, H. B. Khiem, P. K. Sac, A. L. Corwin, K. S. Myint, and D. W. Vaughn. 1997. Detection of immunoglobulin G to the heptatitis E virus among several animal species in Vietnam. Am. J. Trop. Med. Hyg. 57:211 (abstract). 47. Torresi, J., F. Li, S. A. Locarnini, and D. A. Anderson. 1999. Only the nonglycosylated fraction of hepatitis E virus capsid (open reading frame 2) protein is stable in mammalian cells. J. Gen. Virol. 80:1185-1188. 48. Tyagi, S., S. Jameel, and S. K. Lal. 2001. Self-association and mapping of the interaction domain of hepatitis E virus ORF3 protein. J. Virol. 75:2493-2498. 49. Tyagi, S., H. Korkaya, M. Zafrullah, S. Jameel, and S. K. Lal. 2002. The phosphorylated form of the ORF3 protein of hepatitis E virus interacts with its nonglycosylated form of the major capsid protein, ORF2. J. Biol. Chem. 277:22759-22767. 50. van der Poel, W. H, F., Verschoor, R., van der Heide, M. I., Herrera, A., Vivo, M. 109 Kooreman, and A. M. de Roda Husman. 2001. Hepatitis E virus sequences in swine related to sequences in humans, The Netherlands. Emerg. Infect. Dis. 7:970-976. 51. Wang, Y., R. Ling, J. C. Erker, H. Zhang, H. Li, S. Desai, I. K. Mushahwar, and T. J. Harrison. 1999. A divergent genotype of hepatitis E virus in Chinese patients with acute hepatitis. J. Gen. Virol. 80:169-177. 52. Wang, Y., H. Zhang, R. Ling, H. Li, and T. J. Harrison. 2000. The complete sequence of hepatitis E virus genotype 4 reveals an alternative strategy for translation of open reading frames 2 and 3. J. Gen. Virol. 81:1675-1686. 53. Wang, Y. C., H. Y. Zhang, N. S. Xia, G. Peng, H. Y. Lan, H. Zhuang, Y. H. Zhu, S. W. Li, K. G. Tian, W. J. Gu, J. X. Lin, X. Wu, H. M. Li, and T. J. Harrison. 2002. Prevalence, isolation, and partial sequence analysis of hepatitis E virus from domestic animals in China. J. Med. Virol. 67:516-521. 54. Williams, T. P. E., C. Kasorndorkbua, P. G. Halbur, G. Haqshenas, D. K. Guenette, T. E. Toth, and X. J. Meng. 2001. Evidence of extrahepatic sites of replication of the hepatitis E virus in a swine model. J. Clin. Microbiol. 39:3040-3046. 55. Yoo, D., P. Willson, Y. Pei, M. A. Hayes, A. Deckert, C. E. Dewey, R. M. Friendship, Y. Yoon, M. Gottschalk, C. Yason, and A. Giulivi. 2001. Prevalence of hepatitis E virus antibodies in Canadian swine herds and identification of a novel variant of swine hepatitis E virus. Clin. Diagn. Lab. Immunol. 8:1213-1219. 56. Zafrullah, M., M. H. Ozdener, S. K. Panda, and S. Jameel. 1997. The ORF3 protein of hepatitis E virus is a phosphopretein that associates with the cytoskeleton. J. Virol. 71:90459053. 57. Zanetti, A. R., G. G. Schlauder, L. Romano, E. Tanzi, P. Fabris, G. J. Dawson, and I. K. Mushahwar. 1999. Identification of a novel variant of hepatitis E virus in Italy. J. Med. Virol. 57:356-360. 110 6xHis Xpress BamH I PT7 AHEV ORF3 EcoR I pUC pRSET C/AHEV ORF3 3161 bp f1 ori Ampicillin Fig. 5.1. Genetic map of plasmid pRSET C/AHEV ORF3. Avian HEV ORF3 gene with flanking BamH I and EcoR I sites were ligated into the MCS of pRSET C vector, downstream of T7 promoter and in-frame with the sequences of His tag and Xpress epitope. 111 6xHis Xpress BamH I PT7 SHEV ORF3 EcoR I pUC pRSET C/SHEV ORF3 3266 bp f1 ori Ampicillin Fig. 5.2. Genetic map of plasmid pRSET C/SHEV ORF3. Swine HEV ORF3 gene with flanking BamH I and EcoR I sites were ligated into the MCS of pRSET C vector, downstream of T7 promoter and in-frame with the sequences of His tag and Xpress epitope. 112 A B ORF3 MW MW 3 4 6 8 12 15 18 20 MW 5000 bp 3000 bp 2000 bp 1000 bp 264 bp 500 bp 200 bp 100 bp Fig. 5.3. Cloning of avian HEV ORF3 gene. A. Amplified PCR product of avian HEV ORF3 gene with the expected size of 264 bp. B. Positive clones of avian HEV ORF3 recombinant plasmids digested with BamH I and EcoR I. The upper band is the linearized pRSET C vector with the size of 2897 bp (arrow), and the lower band is the avian HEV ORF3 insert with the expected size of 264 bp (arrowhead). 113 A B ORF3 MW MW 1 4 5 7 11 14 17 20 MW 5000 bp 3000 bp 2000 bp 1000 bp 369 bp 500 bp 200 bp 100 bp Fig. 5.4. Cloning of swine HEV ORF3 gene. A. Amplified PCR product of swine HEV ORF3 gene with the expected size of 369 bp. B. Positive clones of swine HEV ORF3 recombinant plasmids digested with BamH I and EcoR I. The upper band is the linearized pRSET C vector with the expected size of 2897 bp (arrow), and the lower band is the swine HEV ORF3 insert with the expected size of 369 bp (arrowhead). 114 Fig. 5.5. A MW 0h 1h 2h 3h 4h MW 0h 1h 2h 3h 4h 3 4 5 5h 6h S P 50 kD 37 kD 25 kD 20 kD 15 kD 10 kD B 5h 6h S P 25 kD 20 kD 15 kD 10 kD C MW 1 2 37 kD 25 kD 20 kD 15 kD 10 kD 115 6 7 8 9 Fig. 5.5. Expression of ORF3 proteins of avian HEV and swine HEV. A. Time course of avian HEV ORF3 protein expression after IPTG induction. The expression was confirmed by Western blot analysis using anti-His G antibody. Arrow indicates the major fraction of the expressed avian HEV ORF3 protein (approximately 12 kDa in size) in E. coli. Arrowhead indicates the dimerization of the expressed avian HEV ORF3 protein with the size of approximately 26 kDa. S represents the soluble protein in the supernatant of cell lysates; P represents the purified avian HEV ORF3 protein (5 µg). Molecular maker (M) is indicated. B. Time course of swine HEV ORF3 protein expression after IPTG induction. The expression was confirmed by Western blot using anti-His G antibody. Arrow indicates the major fraction of the expressed swine HEV ORF3 protein in E. coli. with the size of approximately 15 kDa. S represents the soluble protein in the supernatant of cell lysates; P represents the purified swine HEV ORF3 protein (5 µg). Molecular maker (M) is indicated. C. Expression and purification of swine HEV ORF3 protein. Arrow indicates the major part of the purified swine HEV ORF3 protein with the size of approximately 15 kDa. Arrowhead indicates the dimerization of the purified swine HEV ORF3 protein with the size of approximately 30 kDa. 116 Fig. 5.6. B A MW 75 kD 100 kD 150 kD 250 kD 10 kD 15 kD 75 kD 50 kD 37 kD 25 kD 20 kD S3 MW H3 S3 S3 117 Swine HEV convalescent serum H3 Swine HEV convalescent serum A3 Rabbit antihuman HEV ORF3 serum H3 Mouse anti-avian HEV ORF3 serum A3 Anti-human HEV ORF3 monkey serum Avian HEV convalescent serum A3 S3 Mouse antiswine HEV ORF3 serum H3 Rabbit antihuman HEV ORF3 serum A3 S3 Avian HEV convalescent serum H3 Mouse antiswine HEV ORF3 serum A3 S3 Mouse antiavian HEV ORF3 serum H3 Mouse antiHis G Ab A3 Fig. 5.6. Antigenic cross-reactivity between the ORF3 proteins of avian, swine and human HEVs. A. Western blot analyses of ORF3 proteins of avian and swine HEVs with avian, swine HEV convalescent sera and mono-specific ORF3 antibodies against avian and swine HEVs, human HEV ORF3 antibody as well as anti-His G antibody. A3, avian HEV ORF3 protein. S3, swine HEV ORF3 protein. B. Western blot analyses of human HEV ORF3 protein with anti-human HEV ORF3 antibodies, swine and avian HEV convalescent sera, as well as with anti-swine HEV and anti-avian HEV ORF3 mouse antibodies. H3, human HEV ORF3 protein. 118 Recombinant avian HEV ORF3 protein Avian HEV virion Recombinant avian HEV ORF2 protein Avian HEV virion MW 250 kD 150 kD 100 kD 75 kD 50 kD 37 kD 25 kD 20 kD 15 kD 10 kD Rabbit anti-avian HEV ORF2 peptide serum Mouse antiavian HEV ORF3 serum Fig. 5.7. Characterization of avian HEV virion proteins. Western blot analyses of native virion proteins of avian HEV with anti-avian HEV ORF2 and ORF3 antibodies. 119 Recombinant swine HEV ORF3 protein Swine HEV virion Recombinant swine HEV ORF2 protein Swine HEV virion Recombinant swine HEV ORF3 protein Recombinant swine HEV ORF2 protein Swine HEV virion MW 250 kD 150 kD 100 kD 75 kD 50 kD 37 kD 25 kD 20 kD 15 kD 10 kD Pig anti-human HEV ORF2 serum Swine HEV convalescent serum Mouse antiswine HEV ORF3 serum Fig. 5.8. Characterization of swine HEV virion proteins. Western blot analyses of native virion proteins of swine HEV with swine HEV convalescent serum, anti-human HEV (Sar-55) ORF2 and anti-swine HEV ORF3 antibodies. 120 0(0)/2* 0(0)/4 0(0)/2 0(0)/4 0(0)/2 0(0)/4 0(0)/2 0(0)/4 0(0)/2 0(0)/4 Contact Inocula Contact Inocula Contact Inocula Contact Inocula Contact 0 Inocula Group 0(0)/4 0(0)/2 0(0)/4 0(0)/2 0(0)/4 0(0)/2 0(0)/4 0(0)/2 0(0)/4 0(0)/2 2 0(0)/4 0(0)/2 0(0)/4 1(0)/2 0(0)/4 2(0)/2 0(0)/4 2(0)/2 0(0)/4 2(1)/2 4 0(0)/4 0(0)/2 0(0)/4 1(0)/2 0(0)/4 2(0)/2 0(0)/4 2(0)/2 0(0)/4 2(0)/2 6 121 0(0)/4 0(1)/2 0(0)/4 1(0)/2 0(0)/4 2(0)/2 0(0)/4 2(0)/2 2(0)/4 2(0)/2 8 No. seropositive / no. tested Weeks post-inoculation (WPI) * No. positive for ORF2 antibody (or ORF3 antibody in parentheses) / no. tested. 10-6 10-5 10-4 10-3 10-2 Avian HEV stock dilution 0(0)/4 0(1)/2 0(0)/4 1(0)/2 0(1)/4 2(1)/2 0(1)/4 2(1)/2 2(1)/4 2(0)/2 10 0(0)/4 0(1)/2 0(1)/4 1(1)/2 0(0)/4 2(1)/2 0(1)/4 2(1)/2 2(2)/4 2(1)/2 12 Table 5.1. Seroconversion to avian HEV ORF2 and ORF3 antibodies in chickens experimentally infected with avian HEV CHAPTER VI Evidence of A Hepatitis E Virus (HEV)-Like Agent in Wild Mice in the United States and Attempt to Experimentally Infect Wistar Rats with Strains of HEVs Recovered from A Human, A Pig, and A Chicken Z. F. Sun, and Others (Co-authors To Be Determined) To Be Submitted. 6.1 Abstract The prevalence of IgG anti-HEV antibody was determined in field mice caught in chicken farms to assess the possibility of mice as a potential reservoir for HEV infection in chickens. Three different recombinant HEV antigens derived from avian HEV, swine HEV, and human HEV were used in the ELISA assays. The anti-HEV seropositive rates in wild mice (Mus musculus) were 15/76 (20%), 39/74 (53%), and 43/74 (58%), respectively, depending upon the antigen used in the ELISA. HEV RNA was also detected from 29 fecal and/or serum samples of the mice. The HEV sequences recovered from mice shared 72-100% nucleotide sequence identities with each other, 73-99% sequence identities with isolates recovered from chickens with HS syndrome and 51-60% sequence identities with selected representative strains of swine and human HEV. However, attempts to experimentally infect laboratory mice (Mus musculus) with the PCR-positive fecal material from the wild field mice were unsuccessful. We also experimentally inoculated 10 Wistar rats each with avian HEV, swine HEV, and an US-2 strain of human HEV. However, the rats did not become infected as evidenced by the lack of viremia, virus shedding in feces or seroconversion. These data suggest that mice caught in chicken farms are infected by a HEV-like virus, but additional work is needed to determine the origin of the mouse virus as well as the potential role of rodents in HEV transmission. 122 6.2 Introduction Hepatitis E is an important public health problem in many developing countries and also endemic in some industrialized countries, including the United States (1, 5, 6-7, 9, 16, 2122, 28, 30-32, 41, 48). The disease primarily affects young adults and has a high mortality rate (up to 20%) in infected pregnant women (7, 12, 30-31). Hepatitis E is usually transmitted by fecal-oral route, often through contaminated drinking water (2, 4). The causative agent of the disease, hepatitis E virus (HEV), is a positive sense, single-stranded RNA virus without an envelope (5). The viral genome is about 7.2 kb, which contains three open reading frames (ORFs). ORF1 encodes nonstructural proteins, ORF2 encodes a major putative capsid protein and ORF3 encodes a small phosphorylated protein (1, 5, 7). Due to the lack of a practical animal model and a reliable cell culture system for HEV, studies on HEV replication and pathogenesis have been hampered. The first animal strain of HEV, swine HEV, was isolated in the U.S. in 1997 (23). Subsequently, swine HEV isolates have been identified from pigs worldwide (10, 19, 28-29, 38-39, 43-44, 47). Swine HEV is antigenically and genetically related with human HEV (14). More recently, a second animal strain of HEV, avian HEV, was identified in the United States from chickens with hepatitissplenomegaly (HS) syndrome (13, 33-34). Avian HEV was also identified from apparently healthy chickens (36). It has been shown that a genotype 3 human HEV can cross species barriers and experimentally infect pigs, and conversely swine HEV infects non-human primates (11, 24, 27, 45). Avian HEV also can cross species barriers and infect turkeys (37). Recently, studies in the United States as well as in other countries showed that veterinarians and other pig handlers are at increased risk of zoonotic HEV infection (26, 46). The reported transmission of HEV from deer to humans provided direct evidence of zoonotic transmission (38, 40). Rodents (mice and rats) are commonly found in proximity to chicken farms. Since avian HEV infection in chickens is widespread, the objectives of this study are to investigate the potential role of mice in avian HEV transmission, and to assess the susceptibility of laboratory rats to HEV infection. 123 6.3 Materials and Methods 6.3.1 Field Mouse Study 6.3.1.1 Trapping and Sample Collection of Field Mice in Chicken Farms A total of ninety house mice (Mus musculus) were trapped inside eight different chicken houses on a poultry farm in Virginia during the period from July 2 to October 18, 2002 (Table 6.1). Mice were weighed to estimate the age. Blood samples were collected from 76 mice by retro orbital sinus puncture under anesthesia, and the sera were stored at -80°C. Fecal samples were collected from 87 mice during necropsy. Fecal samples were diluted in sterile PBS (1:10 w/v) and clarified by centrifugation at 3,000 rpm for 15 min at 4°C. The fecal suspensions were harvested and stored at -80°C. 6.3.1.2 ELISA to Detect IgG Anti-HEV Antibodies in Field Mice Purified recombinant ORF2 capsid proteins of avian HEV (expressed in E. coli), swine HEV (expressed in a baculovirus), and human HEV (Sar-55 strain) were used as the ELISA antigens to test the mouse sera (26, 35-36, 42). Rat serum immunized with the recombinant capsid protein of the Sar-55 strain of human HEV, convalescent sera from a pig and a chicken experimentally infected with swine HEV and avian HEV, respectively, were used as positive controls. Pre-inoculation laboratory rat and mouse sera as well as pre-inoculation pig and chicken sera were included as negative controls, respectively. The cut-off values for avian HEV, and swine HEV and human HEV antigens were 0.300, which equaled the mean values of the negative specimens plus 3 times of standard deviation. All serum samples were tested in duplicate. 6.3.1.3 RT-PCR to Detect HEV-Like Sequences from Mouse Samples Primers used for the genetic identification of HEV-like sequences from field mice were designed from the helicase gene region in ORF1 based on multiple sequence alignments of avian HEV and other HEV isolates (16, 35). Two nested sets of degenerate primers were used: external primer set, Helic F: 5’-TGGCGCACC(T)GTA(T)TCC(T)CACCG-3’; Helic R: 5’-CCTCA(G)TGGACCGTA(T)ATCGACCC-3’; internal primer set, Helic F-2: 5’- 124 CACCG(C)TTGCCC(C)TTGGGAC(T)GT-3’; Helic R-2: 5’GACCCA(G)GGA(G)TTCGACTGCTT-3’. The sizes of expected PCR products for the first and second rounds of PCR are 187 bp and 155 bp, respectively. For confirmation purpose, positive samples from the field mice were re-tested with an additional nested set of degenerate primers: external primer set, AHEV F-1/SD: 5’TGTTATT(C)ACACCCACCAAG(A)ACGT(C)TG-3’; Helic R: 5’CCTCA(G)TGGACCGTA(T)ATCGACCC-3’; internal primer set, AHEV F-2/SD: 5’GCCACGGCTG(A)TTACACCC(T)CAC(T)GT-3’; Helic R-2: 5’GACCCA(G)GGA(G)TTCGACTGCTT-3’. The sizes of expected PCR products for the first and second rounds of PCR were 452 bp and 386 bp, respectively. 6.3.1.4 Sequence Comparison and Phylogenetic Analyses Positive PCR products were excised from a 0.8% agarose gel, purified using the GENECLEAN III kit (Q•BIOgene, BIO 101 Systems), and directly sequenced at the Virginia Bioinformatics Institute Core Laboratory Facility with an automated DNA Sequencer. The PCR primer sequences were excluded from the resulting sequences. Only 115 bp of the resulting 155 bp (for the first PCR), and 343 bp of the resulting 386 bp (for the confirmation PCR) helicase gene sequences were used for comparison with the corresponding regions of avian HEV isolates, as well as with selected known representative strains of swine and human HEV by the MacVector computer program (Oxford Molecular Inc.). Phylogenetic analyses were conducted with the aid of the PAUP program (David L. Swofford, Smithsonian Institute, Washington DC, USA). The branch-and-bound search and mid-point rooting options with 1,000 replicates were used to generate phylogenetic trees. 6.3.2 Attempts to Experimentally Infect Laboratory Mice with PCRPositive Fecal Materials Recovered from Wild Field Mice 6.3.2.1 Animals Since the mice trapped on the chicken farm were identified as house mice (Mus musculus), the SF/CamEi strain of wild-derived inbred mice (Mus musculus) (The Jackson Laboratory, Bar Harbor, ME) were used for the transmission study. Three pairs of weanling 125 mice (Species: Mus musculus; Strain: SF/CamEi; Generation: F94; Stock Number: 000280) were purchased from the Jackson Laboratory and used for breeding at Virginia Tech animal facility. Due to cannibalism by this particular species of mice, only seven mice were successfully bred from the three pairs, and the seven mice were available for the first transmission study. 6.3.2.2 Experimental Design One mouse was injected I.V. with 0.2 ml of PBS as negative control. Two were inoculated I.V. with 0.2 ml of 10% fecal suspension from a PCR-positive field mouse (M42), two were inoculated I.V. with 0.2 ml of 10% fecal suspension from another PCR-positive field mouse (M46). Two were inoculated I.V. with 104.5 CID50 of an avian HEV infectious stock (37). Weekly fecal samples, and weekly (first 4 weeks) or biweekly (from 4 weeks onwards) serum samples were collected from each mouse and tested for evidence of HEV infection. A second mouse transmission experiment was carried out using the six breeding mice. One mouse was inoculated I.V. with 0.2 ml of PBS as the control. Three mice were each injected I.V. with 0.2 ml of the PCR-positive fecal suspension from one of the three field mice (M4, M19 and M41), respectively. Two mice were each injected I.V. with 104.5 CID50 of an avian HEV infectious stock. In addition, three Balb/c mice (Charles River Laboratories, Wilmington, MA) were each injected I.V. with 104.5 CID50 of the avian HEV infectious stock. Mice were monitored for 15 weeks. Fecal and serum samples were collected weekly or biweekly as previously described and examined by RT-PCR for the presence of viral RNA. Serum samples were tested by ELISA for seroconversion to anti-HEV antibodies. 6.3.3 Attempts to Experimentally Infect Wistar Laboratory Rats with Strains of HEV Recovered from A Human, A Pig, and A Chicken 6.3.3.1 Viruses The swine HEV used in this study was recovered from a pig in Illinois, and had an infectious titer of 103.5 50% pig infectious doses (PID50) per 0.1 ml of inoculum (23, 25). The US-2 strain of human HEV (kindly provided by Dr. Robert Purcell, NIH, Bethesda, MD) had 126 an infectious titer of 2 X 104 50% monkey infectious doses (MID50) per 0.1 ml of inoculum. The avian HEV was isolated from bile samples of chickens with HS syndrome and had an infectious titer of 5 X 103.5 50% chicken infectious doses (CID50) per 0.1 ml of inoculum (13, 37). 6.3.3.2 Experimental Inoculation of Laboratory Rats Forty 4-week-old female specific-pathogen-free (SPF) Wistar rats (Taconic, Germantown, NY) were divided into four groups of 10 each. Each rat was inoculated intravenously via the leg or jugular vein with 0.1 ml of the swine HEV, human HEV or avian HEV, respectively. Ten rats were each inoculated with 0.1 ml of sterile phosphate-buffered saline (PBS) (GIBCO-BRL) as controls. Fecal and serum samples were collected at 0 (preinoculation), 4, 7, 12, 16, 20, 24, 28, 32, 36, 40, 49, 56, 70, and 84 days post-inoculation (DPI) and examined by a nested RT-PCR for the presence of HEV RNA with primers specific for swine HEV, human HEV, and avian HEV. Two rats from each group were necropsied at 12 and 20 DPI, and the remaining rats were necropsied at 84 DPI. Serum, feces, bile and a variety of tissue samples including tonsil, salivary gland, mesenteric lymph nodes, hepatic lymph nodes, lung, thymus, liver, spleen, pancreas, kidney, duodenum, jejunum, ileum, and colon were collected. Fecal samples collected at different time points were re-suspended in 10% (w/v) sterile PBS. The suspensions were clarified by centrifugation at 3,000 rpm for 15 min at 4°C. Supernatants were harvested and stored at -80°C. 6.3.3.3 RT-PCR to Detect HEV RNA in Inoculated Rats For rats inoculated with swine HEV and the US-2 strain of human HEV, a set of degenerate primers were designed in the ORF2 to amplify both the swine HEV and US-2 strain of human HEV. The first round PCR produced an expected fragment of 531 bp using the forward primer SHEV F-1: 5'-TATGATAACCAGCAC(A)GAGCAAGACC-3' and the reverse primer SHEV R-1: 5'-ACGA(G)GCA(G)GGGTAATCAAC(T)AGTATCC-3'. For the second round, the forward primer SHEV F-2: 5'CCCT(C)TTCTCAGTTCTTCG(C)TGCCAAT-3' and the reverse primer SHEV R-2: 5'- 127 A(G)CCAGCCTCCCAAAAGGACAGCTTC-3' were used to produce an expected product of 274 bp. For rats inoculated with avian HEV, a nested RT-PCR was used to detect avian HEV RNA as described previously (17). The first round PCR produced an expected product of 374 bp using the forward primer BLSV-1 (5’-GCTAGGCGACCCGCACCAGAT-3’) and the reverse primer BLSV-2 (5’- GGTTAGCGCAACAATAGCATG-3’). For the second round of PCR, the forward primer Helic F [5’-TGGCGCACC(T)GTA(T)TCC(T)CACCG-3’] and the reverse primer Helic R [5’-CCTCA(G)TGGACCGTA(T)ATCGACCC-3’] were used to amplify an expected product of 187 bp. Total RNA was extracted with TriReagent (Molecular Research Center, Inc.) from 100 µl of rat fecal or serum samples and resuspended in 12.25 µl of DNase-free, RNase-free, and proteinase-free water (GIBCO-BRL). Reverse transcription was performed at 42°C for 60 min in the presence of a master mix consisting of 12.25 µl total RNA, 0.25 µl Superscript II reverse transcriptase (Invitrogen), 1 µl of 10 µM antisense primer, 0.5 µl of RNase inhibitor, 1 µl of 0.1M dithioteritol, 4 µl of 5x RT buffer, and 1 µl of 10 mM dNTPs. The resulting cDNA was amplified by PCR with appropriate primers, and AmpliTaq Gold DNA polymerase (Applied Biosystems). The PCR reaction parameters consisted of an initial incubation at 95°C for 9 min, followed by 39 cycles of 94°C for 1 min, 42°C for 1 min and 72°C for 1.5 min, with a final incubation at 72°C for 7 min. The PCR products were examined on a 0.8% agarose gel. 6.3.3.4 Generation of Rat Antiserum against the ORF2 Capsid Protein of Human HEV In order to generate positive control rat antiserum, two 4-week-old female SPF Wistar rats (Taconic) were immunized intramuscularly with 25 µg of recombinant ORF2 protein of Sar-55 strain of HEV in Freund’s Adjuvant Incomplete (FAI, F-5506) (Sigma-Aldrich, St. Louis, MO). Three booster doses of aqueous protein (without FAI) were given at two-week intervals. Sera were collected prior to immunization and at seven weeks after the initial immunization. Anti-HEV antibody titers were determined by ELISA. Depending on the antibody titer, blood was collected under anesthesia at about 9 weeks post-immunization and the rats were necropsied. 128 6.3.3.5 ELISA to Detect IgG Anti-HEV Antibodies in Inoculated Rats The recombinant capsid protein of Sar-55 strain of human HEV was used as the antigen to detect the IgG anti-HEV in rats inoculated with swine HEV and human HEV (35, 42). The recombinant capsid protein of avian HEV was used as the antigen to detect the antiHEV antibody in rats inoculated with avian HEV (26, 36). The ELISA protocols have been described previously (14, 26, 36). Briefly, rat sera were diluted at 1:100 and added to ELISA plates coated with the respective recombinant proteins (0.05 µg per well). Horseradish peroxidase-conjugated goat anti-rat IgG (diluted 1:1,000, KPL) was the secondary antibody. The cut-off value of OD405 was conservatively set at 0.300, which is the mean value of preinoculation rat sera plus 3 times the standard deviation. Rat serum immunized with the recombinant capsid protein of the Sar-55 strain of human HEV, convalescent sera from a pig and a chicken experimentally infected with swine HEV and avian HEV, respectively, as well as pre-inoculation rat serum were included as positive and negative controls. All serum samples were tested in duplicate. 6.4 Results 6.4.1 Detection of IgG Anti-HEV Antibodies in Field Mice Caught on A Chicken Farm A serological survey for the prevalence of HEV antibodies among field mice trapped in eight different chicken houses on a chicken farm was conducted. Three different HEV antigens were used for the ELISA assays (Figs. 6.1A, 6.1B and 6.1C). The anti-HEV seropositive rates in mice were 15/76 (20%), 39/74 (53%), 43/74 (58%) when tested with avian HEV, swine HEV, and human HEV antigen, respectively. Mice were collected over time from July 2 to October 18, 2002 (Table 6.1). It appears that the number of mice that were seropositive for avian HEV increased with the amount of time the birds and mice were in contact. Either the virus that was isolated from the birds has mutated enough to be "serologically" different from the original virus in the mice or the mice have gotten exposed to the chicken virus over time. The number of mice seropositive for swine HEV and human HEV was also increasing over time, indicating HEV or HEV-like agent was mounting in the 129 field mice during the 3.5 month time course (Fig. 6.1). In general, the antibody positivity rate increased with weight (age) among field mice. The concordance rates among the three HEV antigens were: 38.46% (15/39) between avian HEV and swine HEV antigens, 34.88% (15/43) between avian HEV and human HEV antigens, and 90.70% (39/43) between swine HEV and human HEV antigens. The results from swine HEV and human HEV antigens correlated well. This is not surprising, since swine HEV capsid gene is very closely related to that of human HEV, whereas avian HEV capsid gene is very divergent from human HEV and swine HEV. 6.4.2 Detection of HEV-Like Sequences from Field Mice Caught on A Chicken Farm Mouse fecal and serum samples collected during necropsies were tested by RT-PCR for HEV RNA. Eight of 71 (11.27%) serum and 21 of 87 (24.14%) stool samples were positive by at least one PCR assay. The PCR products amplified were sequenced for a 155 bp helicase region for most isolates, for a 323 bp helicase region for several isolates (M4-F, M19F, M41-F, M42-F, M46-F, M55-S, M70-S and M83-S), and for a 386 bp helicase region for three isolates (M42-F, M46-F and M59-S). The sequences obtained were compared to avian HEV isolates as well as with selected known representative strains of swine HEV and human HEV. Sequence analyses revealed that the HEV-like sequences recovered from mice shared 72-100% nucleotide sequence identity with each other, 76-100% identities with avian HEV identified from a healthy chicken, 73-99% sequence identities with avian HEV isolates from chickens with HS syndrome, and 51-60% sequence identities with representative strains of swine HEV and human HEV (Table 6.2). Surprisingly, the sequences amplified from fecal and serum samples of mice M9 and M63 were different with only 72% and 79% sequence identities, respectively. The sequences from mouse M59 serum and mouse M63 fecal samples were identical to that of avian HEV identified from chickens on the same farm. 130 6.4.3 Phylogenetic Analyses of HEV-Like Sequences from Field Mice Phylogenetic analyses based on the partial ORF1 helicase gene region indicated that the HEV-like sequences recovered from the mice are more closely related to the avian HEV isolates than to swine and human HEVs (Figs. 6.2A and 6.2B). The HEV sequences from the mice clustered in a major branch with avian HEV isolates. However, several minor branches exist among the HEV sequences from mice. In two mice (M9 and M63), HEV sequences were detected both in serum and fecal samples. Mouse M63 also had high level of HEV IgG antibody, suggesting that anti-HEV antibody and viruses co-exist in this mouse. However, with the exception of 5 mice (M34, M37, M38, M69, and M70), all other PCR-positive mice were seronegative. 6.4.4 Failure to Transmit HEV Infection to Laboratory Mice with PCRPositive Fecal Materials Recovered from Field Mice To confirm that the HEV-like sequences recovered from field mice are indeed due to virus infection in field mice, laboratory mice were intravenously inoculated with the PCRpositive fecal materials from two field mice as well as with the prototype strain of avian HEV. However, HEV RNA was not detected by RT-PCR from any of the inoculated or control mice. Sera of the inoculated and control mice were also examined by ELISA (Fig. 6.3). With the exception of 2 mice (#5 and #6), all other inoculated mice remained seronegative throughout the study. Mouse #5 had a transitory rise in IgG anti-HEV from 3-14 DPI, whereas mouse #6 had clear evidence of seroconversion. 6.4.5 Failure to Experimentally Infect Wistar Rats with Human HEV, Swine HEV or Avian HEV It has been reported that Wistar rats can be experimentally infected with fecal material collected from a human hepatitis E patient (20). In this study, we attempted to infect Wistar rats with three well-characterized strains of HEV with known infectious titers: human HEV, swine HEV, and avian HEV. However, evidence of HEV infection was not observed in any 131 rats inoculated with any of these three HEV strains. The inoculated rats had no viremia, fecal virus shedding or seroconversion throughout the study. 6.5 Discussion Avian HEV infection is widespread in commercial chickens (13, 17, 36). Mice are often found in close proximity, thus providing the opportunity for potential cross-species infection. Rodents in the former Soviet Union were reported to be seropositive for anti-HEV antibody (18). In this study, by using three different HEV antigens, we found that wild house mice caught on a chicken farm with ongoing avian HEV infection had relative high prevalence of anti-HEV IgG antibodies, ranging from 20-58%, depending upon the type of HEV antigen used. The results are consistent with report that mice from Georgia are seropositive (8), although others reported that anti-HEV IgG antibody was absent in mice from India, the United States and Japan (3, 15, 46). The serological data from this study suggested that mice on this farm were naturally infected with HEV or a HEV-like agent. HEV-like sequences were identified from the wild field mice caught in the chicken farm. The detection of viremia in mice suggested that the virus is replicating in mice. The close genetic relatedness between the HEV sequences recovered from field mice and avian HEV suggested that avian HEV may infect mice, and that mice could serve as a reservoir for the HEV infection in chickens. Large amounts of viruses are excreted in feces of chickens experimentally and naturally infected with avian HEV (36-37). Therefore, chicken fecescontaminated feed and water, or chicken manure could be a source for avian HEV infection in field mice, as chicken farms are often infested with rodents. The HEV sequences recovered from field mice are very divergent, and are genetically most closely-related to avian HEV isolates. In fact, a few HEV sequences recovered from mice are identical to the sequence of avian HEV circulating in the chicken farm. The chickens on the farm were reared as pullets in different locations (VA, NC, SC) and then relocated to the test farm at approximately 17 weeks of age. Thus, the complex background of chickens in this farm may partially explain the genetic diversity. Surprisingly, the HEV-like sequences recovered from the serum and fecal samples of the same mice in two mice were also very different. It is possible that some mice may get infected by more than one strain of the virus. However, a more likely explanation is that the 132 sequences amplified from the sera represent the true HEV sequence infecting the mice, whereas the sequences detected from feces of mice could be simply due to "pass-through" of avian HEV as a result of consuming chicken feces-contaminated feed or water. The failure to transmit HEV infection to laboratory mice with the PCR-positive mouse fecal materials further suggests that the sequences detected from mouse feces may not be from replicating viruses. The chicken and mouse samples in this study were collected at different times and at different locations: chicken samples were collected on the poultry farm, whereas trapped mice were brought back to our animal facilities, and the mouse samples were collected in the necropsy room. RT-PCR of the chicken samples from this farm was performed two years before the mouse sera were tested. The lab for RNA extraction and PCR assembly is physically separated from the main lab (in two different buildings) where post-PCR manipulations were performed. In addition, the mouse specimens were tested with different sets of primers targeted at different regions. Therefore, it is unlikely that the HEV sequences detected in the mouse fecal and serum samples were due to contamination with chicken materials. Since Wistar rats were reportedly infected by a human HEV (20), we also attempted to experimentally infect Wistar rats with three well-characterized strains of HEVs: avian HEV, swine HEV and human HEV US-2 strain. However, evidence of HEV infection was not detected in any of the inoculated rats. Another group also failed to experimentally infect rats with genotypes 3 and 4 HEV (J. C. Wu, personal communication). The reason for the discrepancy is not clear. It is likely that the virus naturally infecting rats is very different from the known avian, swine and human HEVs. In conclusion, we showed that field mice caught on a chicken farm are infected by HEV strains closely related to avian HEV. However, we were unable to transmit the infection from the field mice to laboratory mice. We also failed to experimentally infect rats with three different strains of HEV. Future studies are warranted to fully characterize the virus infecting mice, and to understand the ecology of avian HEV infection in chicken farms. 133 6.6 Acknowledgements We would like to thank Dr. Nammalwar Sriranganathan, Dr. David M. Moore and Ms. Mary Nickle for their technical assistance in animal care and blood collection, and Mr. Denis K. Guenette for technical assistance. This study is supported by grants from the NIH (AI 01653, AI 46505 and AI 50611) and from the USDA (NRI 35204-12531). 134 6.7 References 1. Aggarwal, R., and K. Krawczynski. 2000. Hepatitis E: an overview and recent advances in clinical and laboratory research. J. Gastroenterol. Hepatol. 15:9-20. 2. Arankalle, V. A., M. S. Chadha, S. A. Tsarev, S. U. Emerson, A. R. Risbud, K. Banerjee, and R. H. Purcell. 1994. Seroepidemiology of water-borne hepatitis in India and evidence for a third enterically transmitted hepatitis agent. Proc. Natl. Acad. Sci. USA 91:3428-3432. 3. Arankalle, V. A., M. V. Joshi, A. M. Kulkarni, S. S. Gandhe, L. P. Chobe, S. S. Rautmare, A. C. Mishra, and V. S. Padbidri. 2001. Prevalence of anti-hepatitis E virus antibodies in different Indian animal species. J. Viral Hepatitis 8:223-227. 4. Balayan, M. S., A. G. Andjaparidze, S. S. Savinskaya, E. S. Ketiladze, D. M. Braginsky, A. P Savinov, and V. F. Poleshchuk. 1983. Evidence for a virus in non-A, non-B hepatitis transmitted via fecal-oral route. Intervirology 20:23-31. 5. Bradley, D. W. 1992. Hepatitis E: epidemiology, etiology and molecular biology. Rev. Med. Virol. 2:19-28. 6. Clemente-Casares, P., S. Pina, M. Buti, R. Jardi, M. MartIn, S. Bofill-Mas, R. Girones. 2003. Hepatitis E virus epidemiology in industrialized countries. Emerg. Infect. Dis. 9:448-454. 7. Emerson, S. U., and R. H. Purcell. 2003. Hepatitis E virus. Rev. Med. Virol. 13:145-154. 8. Favorov, M. O., M. Y. Kosoy, S. A. Tsarev, J. E. Childs, and H. S. Margolis. 2000. Prevalence of antibody to hepatitis E virus among rodents in the United States. J. Infect. Dis. 181:449-455. 9. Fukuda, S., J. Sunaga, N. Saito, K. Fujimura, Y. Itoh, M. Sasaki, F. Tsuda, M. Takahashi, T. Nishizawa, and H. Okamoto. 2004. Prevalence of antibodies to hepatitis E virus among Japanese blood donors: identification of three blood donors infected with a genotype 3 hepatitis E virus. J Med Virol. 73:554-561. 10. Garkavenko, O., A. Obriadina, J. Meng, D. A. Anderson, H. J. Benard, B. A. Schroeder, Y. E. Khudaakov, H. A. Fields, and M . C. Croxson. 2001. Detection and characterization of swine hepatitis E virus in New Zealand. Journal of Medical Virology 65:525-529. 135 11. Halbur, P. G., C. Kasorndorkbua, C. Gilbert, D. Guenette, M. B. Potters, R. H. Purcell, S. U. Emerson, T. E. Toth, and X. J. Meng. 2001. Comparative pathogenesis of infection of pigs with hepatitis E viruses recovered from a pig and a human. J. Clin. Microbiol. 39:918-923. 12. Hamid, S. S., S. M. Jafri, H. Khan, H. Shah, Z. Abbas, and H. Fields. 1996. Fulminant hepatic failure in pregnant women: acute fatty liver or acute viral hepatitis? J. Hepatol. 25:20-27. 13. Haqshenas, G., H. L. Shivaprasad, P. R. Woolcock, D. H. Read, and X. J. Meng. 2001. Genetic identification and characterization of a novel virus related to human hepatitis E virus from chickens with hepatitis-splenomegaly syndrome in the United States. J. Gen. Virol. 82:2449-2462. 14. Haqshenas, G., F. F. Huang, M. Fenaux, D. K. Guenette, F. W. Pierson, C. T. Larsen, H. L. Shivaprasad, T. E. Toth, and X. J. Meng. 2002. The putative capsid protein of the newly identified avian hepatitis E virus shares antigenic epitopes with that of swine and human hepatitis E viruses and the chicken big liver and spleen disease virus. J. Gen. Virol. 83:2201-2209. 15. Hirano, K., X. Ding, T. C. Li, N. Takeda, H. Kawabata, N. Koizumi, T. Kadosaka, I. Goto, T. Masuzawa, M. Nakamura, K. Taira, T. Kuroki, T. Tanikawa, H. Watanabe, and K. Abe. 2003. Evidence for widespread infection of hepatitis E virus among wild rats in Japan. Hepatol. Res. 27:1-5. 16. Hsieh, S. Y., P. Y. Yang, Y. P. Ho, C. M. Chu, Y. F. Liaw. 1998. Identification of a novel strain of hepatitis E virus responsible for sporadic acute hepatitis in Taiwan. J. Med. Virol. 55:300-304. 17. Huang, F. F., G. Haqshenas, H. L. Shivaprasad, D. K. Guenette., P. R. Woolcock., C. T. Larsen, F. W. Pierson, F. Elvinger, T. E. Toth, and X. J. Meng. 2002. Heterogeneity and seroprevalence of the newly identified avian hepatitis E virus from chickens in the United States. J. Clin. Microbiol. 40:4197-4202. 18. Karetnyi, Y. V., D. I. Dzhumalieva, R. K. Usmanov, I. P. Titova, Y. A. Lituak, and M. S. Balayan. 1993. Possible involvement of rodents in the spread of hepatitis E (in Russian). J. Microbiol. Epidemiol. Immunol. 41:52-56. 136 19. Lu, L., J. Drobeniuc, N. Kobylnikov, R. K. Usmanov, B. H. Robertson, M. O. Favorov, and H. S. Margolis. 2004. Complete sequence of a Kyrgyzstan swine hepatitis E virus (HEV) isolated from a piglet thought to be experimentally infected with human HEV. J. Med. Virol. 74:556-562. 20. Maneerat, Y., E. T. Clayson, K. S. A. Myint, G. D. Young, and B. L. Innis. 1996. Experimental infection of the laboratory rats with the hepatitis E virus. J. Med. Virol. 48:121-128. 21. Mast, E. E., I. K. Kuramoto, M. O. Favorov, V. R. Schoening, B. T. Burkholder, C. N. Shapiro, and P. V. Holland. 1997. Prevalence of and risk factors for antibody to hepatitis E virus seroreactivity among blood donors in Northern California. J. Infect. Dis. 176:34-40. 22. McCrudden, R., S. O’Connell, T. Farrant, S. Beaton, J. P. Iredale, and D. Fine. 2000. Sporadic acute hepatitis E in the United Kingdom: an underdiagnosed phenomenon? Gut 46:732-733. 23. Meng, X. J., R. H. Purcell, P. G. Halbur, J. R. Lehman, D. M. Webb, T. S. Tsareva, J. S. Haynes, B. J. Thacker, and S. U. Emerson. 1997. A novel virus in swine is closely related to the human hepatitis E virus. Proc. Natl. Acad. Sci. USA 94:9860-9865. 24. Meng, X. J., P. G. Halhur, M. S. Shapiro, S. Govindarajan, J. D. Bruna, I. K. Mushahwar, R. H. Purcell, and S. U. Emerson. 1998. Genetic and experimental evidence for cross-species infection by the swine hepatitis E virus. J. Virol. 72:9714-9721. 25. Meng, X. J., P. G. Halbur, J. S. Haynes, T. S. Tsareva, J. D. Bruna, R. L. Royer, R. H. Purcell, and S. U. Emerson. 1998. Experimental infection of pigs with the newly identified swine hepatitis E virus (swine HEV), but not with human strains of HEV. Arch. Virol. 143:1405-1415. 26. Meng, X. J., B. Wiseman, F. Elvinger, D. K. Guenette, T. E. Toth, R. E. Engle, S. U. Emerson, and R. H. Purcell. 2002. Prevalence of antibodies to the hepatitis E virus in veterinarians working with swine and in normal blood donors in the United States and other countries. J. Clin. Microbiol. 40:117-122. 27. Meng, X. J. 2003. Swine hepatitis E virus: cross-species infection and risk in xenotransplantation. Curr. Topics Microbiol. Immunol. 278:185-216. 28. Nishizawa, T., M. Takahashi, H. Mizuo, H. Miyajima, Y. Gotanda, and H. 137 Okamoto. 2003. Characterization of Japanese swine and human hepatitis E virus isolates of genotype IV with 99% identity over the entire genome. J. Gen. Virol. 84:1245-1251. 29. Okamoto, H., M. Takahashi, T. Nishizawa, K. Fukai, U. Muramatsu, and A. Yoshikawa. 2001. Analysis of the complete genome of indigenous swine hepatitis E virus isolated in Japan. Biochem. Biophys. Res. Commun. 289:929-936. 30. Pina, S., M. Buti, M. Cotrina, J. Piella, and R. Girones. 2000. HEV identified in serum from humans with acute hepatitis and in sewage of animal origin in Spain. J. Hepatol. 33:826-833. 31. Purcell, R. H. 1996. Hepatitis E virus, p. 2831-2843. In B. N. Fields, D. M. Knipe, and P. M. Howley, et al. (ed.), Fields virology, 3rd ed., vol 2. Lippincott-Raven Publishers, Philadelphia, Pa. 32. Reyes, G. R. 1997. Overview of the epidemiology and biology of the hepatitis E virus, p. 239-258. In R. A. Willson (ed.), Viral hepatitis. Marcel Dekker, Inc., New York, N. Y.36. Purcell, R. H., and S. U. Emerson. 2001. Animal models of hepatitis A and E. Instit. Lab. Animal. Res. J. 42:161-177. 33. Riddell, C. 1997. Hepatitis-splenomegaly syndrome. In Diseases of Poultry, p. 1041. Edited by Calnek B. W., Barnes H. J., Beard C. W., McDougald L. R., Saif Y. M. Iowa: Iowa State University Press. 34. Ritchie, S. J., and C. Riddell. 1991. Hepatitis-splenomegaly syndrome in commercial egg-laying hens. Can. Vet. J. 32:500-501. 35. Robinson, R. A., W. H. Burgess, S. U. Emerson, R. S. Leibowitz, S. A. Sosnovtseva, S. Tsarev, and R. H. Purcell. 1998. Structural characterization of recombinant hepatitis E virus ORF2 proteins in baculovirus-infected insect cells. Protein Expr. Purif. 12:75-84. 36. Sun, Z. F., C. T. Larsen, A. Dunlop, F. F. Huang, F. W. Pierson, T. E. Toth, and X. J. Meng. 2004. Genetic identification of an avian hepatitis E virus (avian HEV) from healthy chicken flocks and characterization of the capsid gene of 14 avian HEV isolates from chickens with hepatitis-splenomegaly syndrome in different geographic regions of the United States. J. Gen. Virol. 85:693-700. 37. Sun, Z. F., C. T. Larsen, F. F. Huang, P. Billam, F. W. Pierson, T. E. Toth, and X. J. Meng. 2004. Generation and infectivity titration of an infectious stock of avian hepatitis E 138 virus (HEV) in chickens and cross-species infection of turkeys with avian HEV. J. Clin. Microbiol. 42:2658-2662. 38. Takahashi, K., N. Kitajima, N. Abe, and S. Mishiro. 2004. Complete or near-complete nucleotide sequences of hepatitis E virus genome recovered from a wild boar, a deer, and four patients who ate the deer. Virology 330:501-505. 39. Takahashi, M., T. Nishizawa, H. Miyajima, Y. Gotanda, T. Iita, F. Tsuda, and H. Okamoto. 2003. Swine hepatitis E virus strains in Japan form four phylogenetic clusters comparable with those of Japanese isolates of human hepatitis E virus. J. Gen. Virol. 84:851-862. 40. Tei, S., N. Kitajima, K. Takahashi, and S. Mishiro. 2003. Zoonotic transmission of hepatitis E virus from deer to human beings. Lancet 362:371-373. 41. Thomas, D. L., P. O. Yarbough, D. Vlahov, S. A. Tsarev, K. E. Nelson, A. J. Saah, and R. H. Purcell. 1997. Seroreactivity to hepatitis E virus in areas where the disease is not endemic. J. Clin. Microbiol. 35:1244-1247. 42. Tsarev, S. A., T. S. Tsareva, S. U. Emerson, A. Z. Kapikian, J. Ticehurst, W. London, and R. H. Purcell. 1993. ELISA for antibody to hepatitis E virus (HEV) based on complete open-reading frame-2 protein expressed in insect cells: identification of HEV infection in primates. J. Infect. Dis. 168:369-378. 43. van der Poel, W. H, F. Verschoor, R. van der Heide, M. I. Herrera, A. Vivo, M. Kooreman, and A. M. de Roda Husman. 2001. Hepatitis E virus sequences in swine related to sequences in humans, The Netherlands. Emerg. Infect. Dis. 7:970-976. 44. Wang, Y. C., H. Y. Zhang, N. S. Xia, G. Peng, H. Y. Lan, H. Zhuang, Y. H. Zhu, S. W. Li, K. G. Tian, W. J. Gu, J. X. Lin, X. Wu, H. M. Li, and T. J. Harrison. 2002. Prevalence, isolation, and partial sequence analysis of hepatitis E virus from domestic animals in China. J. Med. Virol. 67:516-521. 45. Williams, T. P. E., C. Kasorndorkbua, P. G. Halbur, G. Haqshenas, D. K. Guenette, T. E. Toth, and X. J. Meng. 2001. Evidence of extrahepatic sites of replication of the hepatitis E virus in a swine model. J. Clin. Microbiol. 39:3040-3046. 46. Withers, M. R., M. T. Correa, M. Morrow, M. E. Stebbins, J. Seriwatana, W. D. Webster, M. B. Boak, and D. W. Vaughn. 2002. Antibody levels to hepatitis E virus in 139 North Carolina swine workers, non-swine workers, swine, and murids. Am. J. Trop. Med. Hyg. 66:384-388. 47. Wu, J. C., C. M. Chen, T. Y. Chiang, W. H. Tsai, W. J. Jeng, I. J. Sheen, C. C. Lin, and X. J. Meng. 2002. Spread of hepatitis E virus among different-aged pigs: two- year survey in Taiwan. J. Med. Virol. 66:488-492. 48. Zanetti, A. R., G. G. Schlauder, L. Romano, E. Tanzi, P. Fabris, G. J. Dawson, and I. K. Mushahwar. 1999. Identification of a novel variant of hepatitis E virus in Italy. J. Med. Virol. 57:356-360. 140 Fig. 6.1. A 1.2 1 0.8 0.6 Avian HEV ORF2 Ag 0.4 0.2 0 Mouse ID Fig. 6.1. B 1.6 1.4 1.2 1 0.8 Swine HEV ORF2 Ag 0.6 0.4 0.2 0 Mouse ID 141 Fig. 6.1. C 1.6 1.4 1.2 1 Human HEV Sar-55 Ag 0.8 0.6 0.4 0.2 0 Mouse ID Fig. 6.1. Prevalence of anti-HEV IgG antibodies to ORF2 capsid proteins of avian HEV (A), swine HEV (B) and human HEV (C) in 90 field mice (Mus musculus) trapped in a chicken farm. The cut-off values of OD405 were 0.300 for avian HEV antigen and for swine HEV and human HEV antigens as well. Mice were collected over time from July 2 to October 18, 2002. Mice with lower number were collected earlier. It appears that the number of mice that were seropositive for avian HEV increased with the amount of time the birds and mice were in contact. Either the virus that was isolated from the birds has mutated enough to be "serologically" different from the original virus in the mice or the mice have gotten exposed to the chicken virus over time. Mice that had response to avian HEV generally had response to swine HEV and human HEV. The number of mice seropositive for swine HEV and human HEV was also increasing over time. 142 A 5 changes Fig. 6.2. BLSV Australia HEV US2 Swine HEV US HEV Mexico HEVSar-55 HEV T1 HEV US1 CT090A M35-F M46-F 143 5 changes HEV US1 Swine HEV US HEV US2 HEV T1 HEV Sar-55 HEV Mexico M9-S BLSV Australia M50-S Avian HEV US M56-S CA518.5 M42-F CT090A M46-F M42-F M41-F M9-F M63-S M55-S CA697C CA697B CA697A WI966G CA077 M63-F WI318B M59-S 2553-28F 2553-27F 2553-26S 2553-26F CA242 B M41-F M4-F M9-F M34-F M19-F CA697C CA697B CA697A CA077 CA518.5 CA242 Avian HEV-US WI318B WI966G 2553-28F 2553-27F 2553-26S 2553-26F Fig. 6.2. Phylogenetic tree based on the nucleotide sequences of a 182 bp region (A) and a 115 bp region (B) of the helicase gene of HEV sequences from field mice, avian HEV isolates and Australian chicken big liver and spleen disease virus (BLSV), as well as known representative strains of swine and human HEVs. The tree was constructed with the aid of the PAUP program. A branch-and-bound search with 1,000 replicates and a mid-point rooting option was used to construct the trees. A scale bar representing the numbers of character state changes is proportional to the genetic distance. The HEV sequences detected from the field mice in this study are shown in bold face. Isolates identified from fecal material are annotated with letter F and isolates from serum material are annotated with letter S. 144 0 DPI 3 DPI 1 WPI #1, #4 - AHEV #2, #3 - M42-F #5, #6 - M46-F #7 - PBS 2 WPI 4 WPI 6 WPI Time Post-inoculation 3 WPI 8 WPI 10 WPI 12 WPI 15 WPI #7 #6 #5 #4 #3 #2 #1 Mouse ID 145 post-inoculation (WPI). Seroconversion was observed in mice #5 and #6. The cut-off value of OD405 was set at 0.300. All mice were seronegative at the beginning of the study. Mouse #5 died during routine blood collection process at 2 weeks inoculated with the PCR-positive fecal material of a field mouse M46; and Mouse #7 was injected with PBS as a control. strain; Mice #2 and #3 were inoculated with the PCR-positive fecal material of a field mouse M42; Mice #5 and #6 were from field mice and with avian HEV. Mice #1 and #4 were inoculated intravenously with the prototype avian HEV-US Fig. 6.3. Detection of avian anti-HEV IgG in laboratory mice experimentally inoculated with PCR-positive fecal materials 0 0.1 0.2 0.3 0.4 0.5 0.6 Inocula 7/2/02 1-3 Time Mice Being Trapped Mouse ID Range 4-13 7/19/02 14-21 8/27/02 146 22-27 9/3/02 28-33 9/10/02 34-46 9/17/02 47-59 9/24/02 60-90 10/18/02 Table 6.1. Time at which ninety house mice were trapped inside eight different chicken houses on a poultry farm HEV sequences from field mice Avian HEV isolates from a healthy chicken Prototype avian HEV-US Other avian HEV isolates which cause HS syndrome Chicken BLSV Australia Swine HEV-US HEV US-1 HEV US-2 HEV Sar-55 HEV Mexico HEV T1 147 72-100 76-100 73-99 73-98 74-79 55-58 55-59 55-58 54-57 53-60 51-54 HEV sequences from mice on a chicken farm Table 6.2. Sequence comparison of HEV isolates from field mice in a chicken farm with other known isolates of avian HEV and selected representative strains of swine and human HEV based on a 115 bp region of the helicase gene CHAPTER VII General Conclusions and Future Research Directions Hepatitis E virus (HEV), the causative agent of human hepatitis E, is an important public health problem in many developing countries and is also endemic in many industrialized countries including the United States (1-5, 7-13, 18-20, 24-25, 27-29). The discoveries of avian HEV and swine HEV by our group from chickens and pigs, respectively, and the demonstrated ability of cross-species infection among humans, nonhuman primates and pigs by strains of genotypes 3 and 4 of human HEV and swine HEV, suggest that hepatitis E is a zoonosis (6-7, 14-17, 21-23, 26). We have developed two heteroduplex mobility assays (HMA) to genetically differentiate field strains of avian HEV and swine HEV from known reference strains. It was shown that the HMA profiles generally correlate well with nucleotide sequence identities and with phylogenetic clustering between field strains and the reference swine HEV or avian HEV strains. Therefore, by using different HEV isolates as references, the HMA developed in this study can be used in future studies as a pre-sequencing screening tool to identify variant HEV isolates for further molecular epidemiological studies. We have identified avian HEV from a healthy chicken flock. The avian HEV isolates recovered from the healthy chickens shared 70-97% nucleotide sequence identities with those isolates which cause HS syndrome based on partial helicase and capsid gene regions. The PCR-positive samples from the healthy chickens were successfully used to infect SPF chickens under laboratory conditions, and thus confirming our field results. The capsid gene of avian HEV isolates from chickens with HS syndrome were also characterized and found to be heterogeneic, with 76-100% nucleotide sequence identities to each other. The study indicates that avian HEV is enzootic in chicken flocks and spread subclinically among chicken populations, and that the virus is heterogeneic. Additional experiments are needed to further characterize the avian HEV isolates identified from the apparently healthy chicken flock. The full length sequence of this strain needs to be cloned and sequence comparisons made with other avian HEV isolates which cause HS syndrome. In vivo pathogenesis studies are also needed to determine whether or not the avian HEV strain from the healthy chickens 148 is indeed an avirulent strain. If so, this strain should be evaluated as a potential vaccine candidate. We also prepared an avian HEV infectious stock. The infectivity titer of this infectious stock was determined [5 x 104.5 50% chicken infectious doses (CID50) per ml]. Seroconversion, viremia, and fecal virus shedding were observed in the inoculated chickens. Contact control chickens were also infected via direct contact. Avian HEV infection in chickens was found to be dose-dependent. A cross-species infection was also conducted in one-week old SPF turkeys. The inoculated turkeys seroconverted at 4-8 WPI. Viremia was detected at 2-6 WPI, and fecal virus shedding was identified at 4-7 WPI. This is the first demonstration of cross-species infection by avian HEV. In the future, experiments are warranted to assess the prevalence of HEV antibody in commercial turkeys and to identify the virus responsible for the seropositivity in turkeys. The complete sequence should be determined if the virus can be identified from turkeys. The virus from turkeys should be compared with other HEV isolates identified from other species. We expressed ORF3 proteins of avian and swine HEVs in Escherchia coli, and purified the fusion proteins by BugBuster His-Bind Purification System. Western blot analysis showed that avian HEV ORF3 protein is unique and does not share common antigenic epitopes with those of swine and human HEVs. However, swine HEV (genotype 3) and human HEV (genotype 1) ORF3 proteins cross-react with each other antigenically. We purified virions of avian and swine HEVs by sucrose density gradient centrifugation. We characterized the virion proteins from the purified native virions by SDS-PAGE and Western blot analysis. Two major forms of ORF2 proteins of avian HEV were identified: a 56 kDa and a 80 kDa proteins. Multiple immunoreactive forms of ORF2 proteins of swine HEV were observed: 40 kDa, 53 kDa, 56 kDa and 72 kDa. However, ORF3 protein was not detected from the native virions of avian HEV or swine HEV. These findings provide direct evidence that ORF2 indeed is the structural protein of HEV, whereas ORF3 is not. This is the first report on the analyses of viral proteins of avian and swine HEVs using purified virions. Future studies are needed to determine the structure and function of the ORF3 protein, and the potential interactions between the proteins of ORF2 and ORF3. We determined the prevalence of IgG anti-HEV in field mice caught in chicken farms to assess the possibility of mice as a potential reservoir for HEV infection in chickens. The 149 seropositive rates in wild mice (Mus musculus) are 15/76 (20%), 39/74 (53%), and 43/74 (58%), respectively, depending upon the antigen used in the ELISA. HEV RNA was also detected from 28 fecal and/or serum samples of the mice. The HEV sequences recovered from mice shared 72-100% nucleotide sequence identities with each other, and 73-99% sequence identities with isolates recovered from chickens with HS syndrome. However, attempts to experimentally infect laboratory mice (Mus musculus) with the PCR-positive fecal materials from the field mice were unsuccessful. We also failed to experimentally infect Wistar rats with avian HEV, swine HEV and an US-2 strain of human HEV, respectively. These data suggest that mice caught on chicken farms are infected by a HEV-like virus, but additional work is needed in the future to determine the origin of the mouse virus and to determine the sequence of the entire genome when enough viral materials are available. Efforts should be continued to search for a murine model that can support the replication of HEV. It should also be considered to select some seronegative and HEV or HEV-like agent free wild mice and inoculate them with well-characterized strains of avian HEV, swine HEV and human HEV to assess the susceptibility of wild mice to HEV infection. 150 References 1. Arankalle, V. A., M. O. Favorov, M. S. Chadha, D. M. Phule, and K. Banerjee. 1993. Rhesus monkeys infected with hepatitis E virus (HEV) from the former USSR are immune to subsequent challenge with an Indian strain of HEV. Acta. Virol. 37:515518. 2. Bradley, D. W. 1992. Hepatitis E: epidemiology, etiology and molecular biology. Rev. Med. Virol. 2:19-28. 3. Clemente-Casares, P., S. Pina, M. Buti, R. Jardi, M. Margarita, S. Bofill-Mas, and R. Girones. 2003. Hepatitis E virus epidemiology in industrialized countries. Emerg. Infect. Dis. 9:448-454. 4. Erker, J. C., S. M. Desai, G. G. Schlauder, G. J. Dawson, and I. K. Mushahwar. 1999. A hepatitis E virus variant from the United States: molecular characterization and transmission in cynomolgus macaques. J. Gen. Virol. 80:681-690. 5. Fukuda, S., J. Sunaga, N. Saito, K. Fujimura, Y. Itoh, M. Sasaki, F. Tsuda, M. Takahashi, T. Nishizawa, and H. Okamoto. 2004. Prevalence of antibodies to hepatitis E virus among Japanese blood donors: identification of three blood donors infected with a genotype 3 hepatitis E virus. J Med Virol. 73:554-561. 6. Halbur, P. G., C. Kasorndorkbua, C. Gilbert, D. Guenette, M. B. Potters, R. H. Purcell, S. U. Emerson, T. E. Toth, and X. J. Meng. 2001. Comparative pathogenesis of infection of pigs with hepatitis E viruses recovered from a pig and a human. J. Clin. Microbiol. 39:918-923. 7. Haqshenas, G., H. L. Shivaprasad, P. R. Woolcock, D. H. Read, and X. J. Meng. 2001. Genetic identification and characterization of a novel virus related to human hepatitis E virus from chickens with hepatitis-splenomegaly syndrome in the United States. J. Gen. Virol. 82:2449-2462. 8. Hsieh, S. Y., P. Y. Yang, Y. P. Ho, C. M. Chu, Y. F. Liaw. 1998. Identification of a novel strain of hepatitis E virus responsible for sporadic acute hepatitis in Taiwan. J. Med. Virol. 55:300-304. 9. Huang, C. C., D. Nguyen, J. Fernandez, K. Y. Yun, K. E. Fry, D. W. Bradley, A. W. Tam, G. R. Reyes. 1992. Molecular cloning and sequencing of the Mexico isolate of hepatitis E virus (HEV). Virology 191:550-558. 151 10. Lu, L., J. Drobeniuc, N. Kobylnikov, R. K. Usmanov, B. H. Robertson, M. O. Favorov, and H. S. Margolis. 2004. Complete sequence of a Kyrgyzstan swine hepatitis E virus (HEV) isolated from a piglet thought to be experimentally infected with human HEV. J. Med. Virol. 74:556-562. 11. Maneerat, Y., E. T. Clayson, K. S. A. Myint, G. D. Young, and B. L Innis. 1996. Experimental infection of the laboratory rats with the hepatitis E virus. J. Med. Virol. 48:121-128. 12. Mast, E. E., I. K. Kuramoto, M. O. Favorov, V. R. Schoening, B. T. Burkholder, C. N. Shapiro, and P. V. Holland. 1997. Prevalence of and risk factors for antibody to hepatitis E virus seroreactivity among blood donors in Northern California. J. Infect. Dis. 176:34-40. 13. McCrudden, R., S. O’Connell, T. Farrant, S. Beaton, J. P. Iredale, and D. Fine. 2000. Sporadic acute hepatitis E in the United Kingdom: an underdiagnosed phenomenon? Gut 46:732-733. 14. Meng, X. J., R. H. Purcell, P. G. Halbur, J. R. Lehman, D. M. Webb, T. S. Tsareva, J. S. Haynes, B. J. Thacker, and S. U. Emerson. 1997. A novel virus in swine is closely related to the human hepatitis E virus. Proc. Natl. Acad. Sci. USA 94:9860-9865. 15. Meng, X. J., P. G. Halhur, M. S. Shapiro, S. Govindarajan, J. D. Bruna, I. K. Mushahwar, R. H. Purcell, S. U. Emerson. 1998. Genetic and experimental evidence for cross-species infection by the swine hepatitis E virus. J. Virol. 72:9714-9721. 16. Meng, X. J. 2000. Zoonotic and xenozoonotic risks of hepatitis E virus. Infect. Dis. Rev. 2:35-41. 17. Meng, X. J. 2000. Novel strains of hepatitis E virus identified from humans and other animal species: is hepatitis E a zoonosis? J. Hepatol. 33:842-845. 18. Pina, S., M. Buti, M. Cotrina, J. Piella, and R. Girones. 2000. HEV identified in serum from humans with acute hepatitis and in sewage of animal origin in Spain. J. Hepatol. 33:826-833. 19. Schlauder, G. G., G. J. Dawson, J. C. Erker, P. Y. Kwo, M. F. Knigge, D. L. Smalley, J. E. Rosenblatt, S. M. Desai, I. K. Mushahwar. 1998. The sequence and phylogenetic analysis of a novel hepatitis E virus isolated from a patient with acute hepatitis reported in the United States. J. Gen. Virol. 79:447-456. 152 20. Sun, Z. F., C. T. Larsen, A. Dunlop, F. F. Huang, F. W. Pierson, T. E. Toth, and X. J. Meng. 2004. Genetic identification of an avian hepatitis E virus (avian HEV) from healthy chicken flocks and characterization of the capsid gene of 14 avian HEV isolates from chickens with hepatitis-splenomegaly syndrome in different geographic regions of the United States. J. Gen. Virol. 85:693-700. 21. Sun, Z. F., C. T. Larsen, F. F. Huang, P. Billam, F. W. Pierson, T. E. Toth, and X. J. Meng. 2004. Generation and infectivity titration of an infectious stock of avian hepatitis E virus (HEV) in chickens and cross-species infection of turkeys with avian HEV. J. Clin. Microbiol. 42:2658-2662. 22. Takahashi, K., N. Kitajima, N. Abe, and S. Mishiro. 2004. Complete or near-complete nucleotide sequences of hepatitis E virus genome recovered from a wild boar, a deer, and four patients who ate the deer. Virology 330:501-505. 23. Tei, S., N. Kitajima, K. Takahashi, and S. Mishiro. 2003. Zoonotic transmission of hepatitis E virus from deer to human beings. Lancet 362:371-373. 24. Thomas, D. L., P. O. Yarbough, D. Vlahov, S. A. Tsarev, K. E. Nelson, A. J. Saah, and R. H. Purcell. 1997. Seroreactivity to hepatitis E virus in areas where the disease is not endemic. J. Clin. Microbiol. 35:1244-1247. 25. Tien, N. T., H. T. Clayson, H. B. Khiem, P. K. Sac, A. L. Corwin, K. S. Myint, and D. W. Vaughn. 1997. Detection of immunoglobulin G to the heptatitis E virus among several animal species in Vietnam. Am. J. Trop. Med. Hyg. 57:211 (abstract). 26. Yazaki, Y., H. Mizuo, M. Takahashi, T. Nishizawa, N. Sasaki, Y. Gotanda, and H. Okamoto. 2003. Sporadic acute or fulminant hepatitis E in Hokkaido, Japan, may be foodborne, as suggested by the presence of hepatitis E virus in pig liver as food. J. Gen. Virol. 84:2351-2357. 27. Yoo, D., P. Willson, Y. Pei, M. A. Hayes, A. Deckert, C. E. Dewey, R. M. Friendship, Y. Yoon, M. Gottschalk, C. Yason, and A. Giulivi. 2001. Prevalence of hepatitis E virus antibodies in Canadian swine herds and identification of a novel variant of swine hepatitis E virus. Clin. Diagn. Lab. Immunol. 8:1213-1219. 28. Zafrullah, M., M. H. Ozdener, S. K. Panda, and S. Jameel. 1997. The ORF3 protein of hepatitis E virus is a phosphopretein that associates with the cytoskeleton. J. Virol. 71:9045-9053. 153 29. Zanetti, A. R., G. G. Schlauder, L. Romano, E. Tanzi, P. Fabris, G. J. Dawson, and I. K. Mushahwar. 1999. Identification of a novel variant of hepatitis E virus in Italy. J. Med. Virol. 57:356-360. 154 CHAPTER VIII Characterization of the Severe Acute Respiratory Syndrome (SARS) Coronavirus Nucleocapsid Protein: Identification of Common Antigenic Epitope(s) with Group I Animal Coronaviruses and Implication for SARS Diagnosis Z. F. Sun, and X. J. Meng Journal of Clinical Microbiology, 2004 May;42(5):2351-2352. Modified and re-printed with permission. 8.1 Abstract Severe acute respiratory syndrome (SARS) is an emerging infectious disease associated with a novel coronavirus and causing worldwide outbreaks. SARS coronavirus (SARS-CoV) is an enveloped RNA virus, which contains several structural proteins. Among these proteins, nucleocapsid (N) protein is a major structural protein. In order to develop a diagnostic assay specific for SARS, the SARS-CoV N gene was cloned, expressed in Escherchia coli using a pRSET-C expression vector in this study. After the isopropyl-β-D-thiogalactoside (IPTG) induction, N protein was expressed as an insoluable form and purified by BugBuster His-Bind Purification System. N protein is highly immunogenic and abundantly expressed. The purified recombinant N protein was confirmed with a monoclonal antibody against the Xpress epitope tag and with a convalescent-phase SARS patient serum by Western blot. The antigenic crossreactivity between the N protein of SARS-CoV and that of known animal coronaviruses was analyzed using Western blot. We found that the SARS-CoV N protein shares common antigenic epitope with that of antigenic group I animal coronaviruses but not of antigenic groups II or III, and thus raising concerns for using N protein or whole virus as antigens in serological diagnosis of SARS. The use of SARS-CoV N protein or the whole virus could lead to false positive diagnosis in patients infected by other antigenic group I coronaviruses, and could also 155 complicate the search for an animal reservoir as many animal species are infected by the antigenic group I coronaviruses. 156 8.2 Introduction, Materials and Methods, Results and Discussion Severe acute respiratory syndrome (SARS) is an emerging infectious disease of significant public health concern (3). The causative agent of SARS was shown to be a previously unrecognized virus within the family of Coronaviridae, designated SARSassociated coronavirus (SARS-CoV) (1, 4, 7). There exist three known antigenic groups (I, II, and III) of animal coronaviruses, causing important and severe respiratory and enteric diseases in livestock, poultry and laboratory animals, and common colds (strains 229E and OC43) in humans (3). Sequence analyses revealed that SARS-CoV is not a derivative of any known animal coronaviruses (5, 8). Nevertheless, Ksiazek et al (4) showed that polyclonal antibodies from antigenic group I coronaviruses including human coronavirus 229E, feline infectious peritonitis virus (FIPV), and porcine transmissible gastroenteritis virus (TGEV) reacted with SARS-CoV-infected Vero cells. Since the nucleocapsid (N) proteins of known coronaviruses are relatively conserved, we aimed to determine if the N protein is responsible for the observed antigenic crossreactivity (4). The N gene of the SARS-CoV contains no glycosylation sites (5, 8), and thus we expressed and characterized the N protein in Escherchia coli (Fig. 8.1). Briefly, the N gene was amplified by RT-PCR (Fig. 8.2A) with a set of primers (forward primer 5’CCCGGATCCAAATGTCTGATAATGGACCCC-3’; reverse primer 5’CCCGAATTCTTATGCCTGAGTTGAATCAGC-3’). To facilitate subsequent cloning steps, engineered restriction enzyme (BamH I and EcoR I) sites (underlined) were introduced at the 5' ends of the sense and antisense primers, respectively (Fig. 8.1). The amplified N gene was cloned in-frame with the sequence coding for Xpress™ epitope located upstream of the multiple cloning site of the pRSET-C expression vector (Fig. 8.1). The recombinant plasmids were transformed into E. Coli strain BL21 Star™ (DE3)pLysS cells (Invitrogen) (Fig. 8.2B), which produced T7 polymerase, and the expression of the fusion N protein was driven by a T7 promoter upstream the Xpress™ epitope, and induced by the addition of 1 mM IPTG (isopropyl-β-D-thiogalactopy ranoside) (Fig. 8.3). The SARS-CoV N protein was purified with the BugBuster His-Bind purification kit (Novagen), based on the affinity of His-bind resin for His-tagged fusion N protein, and confirmed with a monoclonal antibody against the fused 157 Xpress epitope (Fig. 8.3) and with a convalescent-phase SARS patient serum (Fig. 8.4) by Western blot. Western blot analysis was used to determine if the N protein of SARS-CoV cross-reacts with polyclonal antisera of known animal coronaviruses (Fig. 8.4). The purified N protein was separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and transferred onto nitrocellulose membranes. After blocking, the membranes were cut into separate strips. The strips were incubated with a 1:100 dilution of a polyclonal antiserum against either human, bovine, swine, chicken, turkey, canine or feline coronaviruses in Trisbuffered saline (TBS) containing 0.05% Tween-20 (Fig. 8.4). After incubation with respective HRP-conjugated secondary antibodies at a 1:1,000 dilution, the immunocomplexes were detected with 4-chloro-1-naphthol (Sigma). The results showed that the N protein of SARSCoV reacted, as expected, with a convalescent-phase SARS patient serum, but it also reacted strongly with polyclonal antisera of known antigenic group I coronaviruses tested in the study including TGEV, FIPV, and canine coronavirus (CCoV), indicating that the N protein of SARS-CoV shares common antigenic epitope(s) with that of antigenic group I animal coronaviruses. The N protein of SARS-CoV, however, does not cross-react with polyclonal antisera from antigenic group II (porcine hemagglutinating encephalomyelitis virus [HEV], and bovine coronavirus [BCoV] or group III (turkey coronavirus [TCoV] and avian infectious bronchitis virus [IBV]) animal coronaviruses tested in our study (Fig. 8.4). The results from this study raised potential concerns for using recombinant N protein of SARS-CoV, whole viral antigen extracts, or virus-infected cells as reagents for diagnosis of SARS-CoV infections in humans and other animal species (2, 4, 9). The antigenic group I coronaviruses are known to infect a variety of animal species, including swine, canines, felines, rabbits, and humans. Thus, the use of native SARS-CoV N protein or whole virus in enzyme-linked immunosorbent assay (ELISA) or indirect immunofluorescence assay (IFA) and the use of SARS convalescent-phase sera or polyclonal antibody raised against native SARS-CoV N protein or whole virus in direct IFA could produce false-positive diagnosis of SARS, although this concern is very minimal since the two known human coronaviruses (strains 229E and OC43) do not cause severe clinical diseases. Although the natural animal reservoir for SARS-CoV has not yet been identified, it is believed that SARS-CoV originated from wild animal species (2, 6). Therefore, the use of N protein or whole virus as diagnostic 158 antigens could also complicate the search for a definitive natural animal reservoir, as many wild and domestic animal species may have already been infected by known group I coronaviruses. Therefore, it is important to identify a specific N protein immunoreactive epitope or other protein specific only for SARS-CoV with no antigenic cross-reactivity to known coronaviruses as the antigen for SARS diagnosis and for identification of SASR-CoV animal reservoir(s). 8.3 Acknowledgements We thank Drs. Dean Erdman, Paul Rota and Thomas Ksiazek of the Centers for Disease Control and Prevention, Atlanta, GA for generously providing SARS-CoV RNA and SARS convalescent-phase patient serum and Dr. F. W. Pierson of Virginia Tech for providing TCoV antibody. We would like to thank Dr. Mohamed Seleem for his expert assistance in protein purification. 159 8.4 References 1. Fouchier, R. A., T. Kuiken, M. Schutten, G. van Amerongen, G. J. van Doornum, B. G. van den Hoogen, M. Peiris, W. Lim, K. Stohr, and A. D. Osterhaus. 2003. Aetiology: Koch's postulates fulfilled for SARS virus. Nature 423:240. 2. Guan, Y., B. J. Zheng, Y. Q. He, X. L. Liu, Z. X. Zhuang, C. L. Cheung, S. W. Luo, P. H. Li, L. J. Zhang, Y. J. Guan, K. M. Butt, K. L. Wong, K. W. Chan, W. Lim, K. F. Shortridge, K. Y. Yuen, J. S. Peiris, and L. L. Poon. 2003. Isolation and characterization of viruses related to the SARS coronavirus from animals in southern China. Science 302:276-278. 3. Holmes, K. V. 2003. SARS coronavirus: a new challenge for prevention and therapy. J. Clin. Invest. 111:1605-1609. 4. Ksiazek, T. G., D. Erdman, C. S. Goldsmith, S. R. Zaki, T. Peret, S. Emery, S. Tong, C. Urbani, J. A. Comer, W. Lim, P. E. Rollin, S. F. Dowell, A. E. Ling, C. D. Humphrey, W. J. Shieh, J. Guarner, C. D. Paddock, P. Rota, B. Fields, J. DeRisi, J. Y. Yang, N. Cox, J. M. Hughes, J. W. LeDuc, W. J. Bellini, L. J. Anderson, and SARS Working Group. 2003. A novel coronavirus associated with severe acute respiratory syndrome. N. Engl. J. Med. 348:1953-1966. 5. Marra, M. A., S. J. Jones, C. R. Astell, R. A. Holt, A. Brooks-Wilson, Y. S. Butterfield, J. Khattra, J. K. Asano, S. A. Barber, S. Y. Chan, A. Cloutier, S. M. Coughlin, D. Freeman, N. Girn, O. L. Griffith, S. R. Leach, M. Mayo, H. McDonald, S. B. Montgomery, P. K. Pandoh, A. S. Petrescu, A. G. Robertson, J. E. Schein, A. Siddiqui, D. E. Smailus, J. M. Stott, G. S. Yang, F. Plummer, A. Andonov, H. Artsob, N. Bastien, K. Bernard, T. F. Booth, D. Bowness, M. Czub, M. Drebot, L. Fernando, R. Flick, M. Garbutt, M. Gray, A. Grolla, S. Jones, H. Feldmann, A. Meyers, A. Kabani, Y. Li, S. Normand, U. Stroher, G. A. Tipples, S. Tyler, R. Vogrig, D. Ward, B. Watson, R. C. Brunham, M. Krajden, M. Petric, D. M. Skowronski, C. Upton, and R. L. Roper. 2003. The genome sequence of the SARS-associated coronavirus. Science 300:1399-1404. 6. Martina, B. E., B. L. Haagmans, T. Kuiken, R. A. Fouchier, G. F. Rimmelzwaan, G. Van Amerongen, J. S. Peiris, W. Lim, and A. D. Osterhaus. 2003. Virology: SARS virus infection of cats and ferrets. Nature 425:915. 160 7. Peiris, J. S., S. T. Lai, L. L. Poon, Y. Guan, L. Y. Yam, W. Lim, J. Nicholls, W. K. Yee, W. W. Yan, M. T. Cheung, V. C. Cheng, K. H. Chan, D. N. Tsang, R. W. Yung, T. K. Ng, K. Y. Yuen, and SARS Study Group. 2003. Coronavirus as a possible cause of severe acute respiratory syndrome. Lancet 361:1319-1325. 8. Rota, P. A., M. S. Oberste, S. S. Monroe, W. A. Nix, R. Campagnoli, J. P. Icenogle, S. Penaranda, B. Bankamp, K. Maher, M. H. Chen, S. Tong, A. Tamin, L. Lowe, M. Frace, J. L. DeRisi, Q. Chen, D. Wang, D. D. Erdman, T. C. Peret, C. Burns, T. G. Ksiazek, P. E. Rollin, A. Sanchez, S. Liffick, B. Holloway, J. Limor, K. McCaustland, M. Olsen-Rasmussen, R. Fouchier, S. Gunther, A. D. Osterhaus, C. Drosten, M. A. Pallansch, L. J. Anderson, and W. J. Bellini. 2003. Characterization of a novel coronavirus associated with severe acute respiratory syndrome. Science 300:1394-1399. 9. Shi, Y., Y. Yi, P. Li, T. Kuang, L. Li, M. Dong, Q. Ma, and C. Cao. 2003. Diagnosis of severe acute respiratory syndrome (SARS) by detection of SARS coronavirus nucleocapsid antibodies in an antigen-capturing enzyme-linked immunosorbent assay. J. Clin. Microbiol. 41:5781-5782. 161 PT7 6xHis Xpress BamH I pUC pRSET C/SARSCoV N 4166 bp SARS-CoV N Genetic Map of Plasmid pRSET C/ Ampicillin SARS-CoV N EcoR I f1 ori Fig. 8.1. Genetic map of plasmid pRSET C/SARS-CoV N. SARS-CoV N gene with flanking BamH I and EcoR I sites were ligated into the MCS of pRSET C vector, downstream of T7 promoter and in-frame with the sequences of His tag and Xpress epitope. 162 A B MW MW 3 N 5000 bp 4 5000 bp 3000 bp 2000 bp 3000 bp 1269 bp 1000 bp 2000 bp 1269 bp 500 bp 1000 bp 200 bp 500 bp 100 bp 200 bp 100 bp Fig. 8.2. Cloning of the nucleocapsid (N) gene of SARS-CoV. A. Amplified PCR product of SARS-CoV N gene with the expected size of 1269 bp. B. Positive clones of SARS-CoV N recombinant plasmids digested with BamH I and EcoR I. The upper band is the linearized pRSET C vector with the size of 2897 bp (arrow), and the lower band is the SARS-CoV N insert with the expected size of 1269 bp (arrowhead). 163 M 0h 1h 2h 3h 4h 5h 6h 7h 8h 9h 10h 18h S P B S P 164 epitope tag fused with the N protein. Molecular maker (M) is indicated. Arrows, 50 kDa. soluble proteins in the supernatant (S) and of the purified protein (P) using monoclonal antibody to the Xpress the supernatant (S) of cell lysates; lane 15, SDS-PAGE of 5 µg of the purified N protein (P). B. Western blot of harvested at 0, 1, 2, 3, 4, 5, 6, 7, 8, 9, 10 and 18 h, respectively, after IPTG induction; lane 14, soluble proteins in Fig. 8.3. A. Expression of the SARS-CoV N protein in E. Coli. Lanes 2-13, SDS-PAGE of bacterial cell lysates 10 kD 15 kD 20 kD 25 kD 37 kD 50 kD 250 kD 150 kD 100 kD 75 kD A II Anti-SARS-CoV (human) III Anti-IBV (CT-46) Anti-TCoV (turkey) Anti-BCoV (calf) Anti-HEV (pig) Anti-FIPV (cat) Anti-CCoV (cat) Anti-TGEV (pig) Anti-TGEV (cat) 165 from VMRD, Inc., Pullman, Wash. The arrow shows the expected size (about 50 kDa) of the SARS-CoV N protein. Laboratories, Ames, Iowa. Polyclonal cat antisera to TGEV and CCoV and cat ascitic fluid against FIPV were purchased antisera to IBV, HEV, BCoV (calf serum), and TGEV (pig serum) were purchased from National Veterinary Service and CCoV), group II (porcine HEV and BCoV), and group III (TCoV and avian IBV) animal coronaviruses. Polyclonal as positive controls. The polyclonal antisera used in the Western Blot analysis were from antigenic group I (FIPV, TGEV, blocking solution. A convalescent-phase SARS human patient serum (anti-SARS-CoV) and anti-Xpress antibody were used animal coronaviruses. SARS-CoV N protein (1µg/lane) was separated by SDS-PAGE. Each antiserum was diluted 1:100 in Fig. 8.4. Western blot analyses of antigenic cross-reactivity of SARS-CoV N protein with polyclonal antisera of known 37 kD 50 kD 75 kD MW Polyclonal Antibodies I Anti-IBV (Beaudette) Antigenic Groups Anti-Xpress epitope tag CURRICULUM VITAE Zhifeng Sun Office: Center for Molecular Medicine and Infectious Diseases College of Veterinary Medicine, Virginia Polytechnic Institute and State University 1410 Price’s Fork Road, Blacksburg, VA 24061-0342 Phone: (540) 231-4339 E-mail: [email protected] Home: 1226 University City Blvd., E-55, Blacksburg, VA 24060-3189 EDUCATION Aug., 2000-Jan., 2005: Ph.D., Molecular Virology Mentor: Dr. Xiang-Jin Meng College of Veterinary Medicine Virginia Polytechnic Institute and State University Blacksburg, VA 24061-0342 Sep., 1989-Jul., 1992: M.S., Veterinary Virology and Immunology Mentors: Dr. Nianxing Du and Dr. Weiyan Xu College of Veterinary Medicine, Nanjing Agricultural University Nanjing, 210095 P. R. China Sep., 1983-Jul., 1987: D.V.M., Veterinary Medicine College of Veterinary Medicine, Henan Agricultural University Zhengzhou, 450002 P. R. China PROFESSIONAL EXPERIENCE Aug., 2000-Jan., 2005: Graduate Research Assistant Center for Molecular Medicine and Infectious Diseases Department of Biomedical Sciences and Pathobiology College of Veterinary Medicine, Virginia Polytechnic Institute and State University Blacksburg, VA 24061 1998-2000: Visiting Scientist Virus and Prion Diseases of Livestock Research Unit National Animal Disease Center, USDA, ARS Ames, IA 50010 166 1997-1998: Senior Research Associate 1993-1997: Research Associate 1992-1993: Research Assistant Research Unit of Swine Viral Diseases Institute of Animal Husbandry and Veterinary Medicine Shanghai Academy of Agricultural Sciences Shanghai, 201106 P. R. China 1990-1992: Graduate Research Assistant 1989-1991: Graduate Teaching Assistant College of Animal Medical Science, Nanjing Agricultural University Nanjing, 210095 P. R. China Sep., 1986-Jun., 1987: Internship 1. Xinzheng Veterinary Hospital, Zhengzhou, 451100 P. R. China 2. Luohe Veterinary Hospital, Luohe, 462000 P. R. China 3. Baima Veterinary Hospital, Dancheng, Zhoukou, 477100 P. R. China SELECTED PEER-REVIEWED PUBLICATIONS 1. Sun Z. F., and Co-authors (To be determined). 2005. Characterization of the ORF3 proteins of human, swine and avian hepatitis E viruses (HEV): identification of antigenic cross-reactivity between swine HEV and human HEV but failure to detect the ORF3 protein in native virions. Journal of Virology (To be submitted). 2. Sun Z. F., and Co-authors (To be determined). 2005. Evidences of a HEV-like agent in wild mice in the United States and attempt to experimentally infect Wistar rats with strains of hepatitis E viruses recovered from a human, a pig and a chicken (To be submitted). 3. Billam P., Huang F. F., Sun Z. F., Guenette D. K., Pierson F. W., Duncan R. B., Elvinger F., Toth T. E., and Meng X. J. 2005. Systematic pathogenesis and replication of avian hepatitis E virus in specific-pathogen-free adult chickens. Journal of Virology, 79(6) (March issue, In Press). 4. Sun Z. F., Larsen C. T., Huang F. F., Billam P., Pierson F. W., Toth T. E., and Meng X. J. 2004. Generation and infectivity titration of an infectious stock of avian hepatitis E virus (HEV) in chickens and cross-species infection of turkeys with avian HEV. Journal of Clinical Microbiology, 42(6):2658-2662. 5. Sun Z. F., and Meng X. J. 2004. Antigenic cross-reactivity between the nucleocapsid protein of severe acute respiratory syndrome (SARS) coronavirus and polyclonal antisera of antigenic group I animal coronaviruses: Implication for SARS diagnosis. Journal of Clinical Microbiology, 42(5):2351-2352. 6. Huang F. F., Sun Z. F., Emerson S.U., Purcell R. H., Shivaprasad H. L., Pierson F. W., Toth T. E., and Meng X. J. 2004. Determination and analysis of the complete genomic sequence of avian hepatitis E virus (avian HEV) and attempts to infect rhesus monkeys with avian HEV. Journal of General Virology, 85(Pt 6):1609-1618. 167 7. Sun Z. F., Larsen C. T., Dunlop A., Huang F. F., Pierson F. W., Toth T. E., and Meng X. J. 2004. Genetic identification of avian hepatitis E virus (HEV) from healthy chicken flocks and characterization of the capsid gene of 14 avian HEV isolates from chickens with hepatitis-splenomegaly syndrome in different geographic regions of the United States. Journal of General Virology, 85(Pt 3):693-700. 8. Sun Z. F., Huang F. F., Halbur P. G., Schommer S. K., Pierson F. W., Toth T. E., and Meng X. J. 2003. Use of heteroduplex mobility assays (HMA) for pre-sequencing screening and identification of variant strains of swine and avian hepatitis E viruses. Veterinary Microbiology, 96(2):165-176. 9. Cheung A. K., Chen Z., Sun Z. F., and McCullough D. 2000. Pseudorabies virus induces apoptosis in tissue culture cells. Archives of Virology, 145(10):2193-2200. 10. Sun Z. F., Du N. X., and Xu W. Y. 1998. Detection of rabbit hemorrhagic disease virus by enzyme-linked immunosorbent assay using monoclonal antibodies. Chinese Journal of Veterinary Science, 18(4):334-337. 11. Sun Z. F., Qian Y. Q., Fang X. L., and Wen R. 1998. Neutralization and passive protection tests of monoclonal antibodies against porcine epidemic diarrhea virus. Chinese Journal of Veterinary Science, 18(3):221-223. 12. Sun Z. F., Qian Y. Q., Fang X. L., and Wen R. 1998. Development and characterization of monoclonal antibodies against porcine epidemic diarrhea virus. Chinese Journal of Veterinary Science, 18(2):131-134. 13. Sun Z. F., Du N. X., and Xu W. Y. 1997. Analysis of the combining epitopes of monoclonal antibodies on rabbit hemorrhagic disease virus. Journal of Nanjing Agricultural University, 20(1):68-72. 14. Sun Z. F., Qian Y. Q., Fang X. L., and Wen R. 1997. Study on new immune reagent against porcine epidemic diarrhea virus. Acta Agriculturae Shanghai, 13(1):90-93. 15. Shan S. H., Hu Q. L., and Sun Z. F. 1997. Isolation and characterization of serotype I Marek’s disease virus. Acta Agriculturae Universitatis Henanensis, 31(2):183-186. 16. Sun Z. F., Du N. X., and Xu W. Y. 1993. A reverse passive hemagglutination test for detection of rabbit hemorrhagic disease virus using monoclonal antibodies. Journal of Nanjing Agricultural University, 16(Suppl.):95-98. SELECTED NON-REFERRED PUBLICATIONS 1. Sun Z. F., and Meng X. J. Characterization of the severe acute respiratory syndrome (SARS) coronavirus nucleocapsid protein: Identification of common antigenic epitope(s) with group I animal coronaviruses and implication for SARS diagnosis. Proceedings of the 85th Conference of Research Workers in Animal Diseases. November 14-16, 2004. #214. Chicago, IL. 2. Guo H., Zhou E-M., Sun Z. F., and Meng X. J. Species specific and non-specific epitope mapping of avian hepatitis E virus capsid protein for differential diagnosis of hepatitis E virus infection. Proceedings of the 85th Conference of Research Workers in Animal Diseases. November 14-16, 2004. #129. Chicago, IL. 3. Sun Z. F., and Meng X. J. Characterization of the severe acute respiratory syndrome (SARS) coronavirus nucleocapsid protein: Identification of common antigenic epitope(s) 168 with group I animal coronaviruses and implication for SARS diagnosis. Proceedings of the 16th Annual Research Symposium, VMRCVM, June 17-18, 2004. 4. Sun Z. F., Larsen C. T., Huang F. F., Billam P., Pierson F. W., Toth T. E., and Meng X. J. Cross-species infection of turkeys with avian hepatitis E virus (HEV). Proceedings of the 16th Annual Research Symposium, VMRCVM, June 17-18, 2004. 5. Billam P., Huang F. F., Sun Z. F., Pierson F. W., Duncan R. B., Elvinger F., Guenette D. K., Toth T. E., and Meng X. J. Systematic pathogenesis and replication of a strain of the hepatitis E virus (HEV) in its natural host: Avian HEV infections in specific-pathogenfree adult chickens. Proceedings of the 16th Annual Research Symposium, VMRCVM, June 17-18, 2004. 6. Sun Z. F., and Meng X. J. Characterization of the severe acute respiratory syndrome (SARS) coronavirus nucleocapsid protein: Identification of common antigenic epitope(s) with group I animal coronaviruses and implication for SARS diagnosis. Proceedings of the 20th Annual Graduate Student Assembly Research Symposium of Virginia Polytechnic Institute and State University, Blacksburg, March 23, 2004. 7. Sun Z. F., Larsen C. T., Huang F. F., Billam P., Pierson F. W., Toth T. E., and Meng X. J. Generation and infectivity titration of an infectious stock of avian hepatitis E virus (HEV) in chickens and cross-species infection of turkeys with avian HEV. Proceedings of the 20th Annual Graduate Student Assembly Research Symposium of Virginia Polytechnic Institute and State University, Blacksburg, March 23, 2004. 8. Huang F. F., Sun Z. F., Pierson F. W., Toth T. E., and Meng X. J. Characterization and analyses of the complete genomic sequence of avian hepatitis E virus (avian HEV). Proceedings of the 20th Annual Graduate Student Assembly Research Symposium of Virginia Polytechnic Institute and State University, Blacksburg, March 23, 2004. 9. Billam P., Huang F. F., Sun Z. F., Guenette D. K., Pierson F. W., Duncan R. B., Toth T. E., and Meng X. J. Systematic pathogenesis and replication of a strain of the hepatitis E virus (HEV) in its natural host: avian HEV infections in specific-pathogen-free adult chickens. Proceedings of the 20th Annual Graduate Student Assembly Research Symposiumof Virginia Polytechnic Institute and State University, Blacksburg, March 23, 2004. 10. Sun Z. F., Larsen C. T., Huang F. F., Billam P., Pierson F. W., Toth T. E., and Meng X. J. Generation and infectivity titration of an infectious stock of avian HEV and crossspecies infection of turkeys with avian HEV. Proceedings of the 84th Conference of Research Workers in Animal Diseases, #169. Chicago, IL, November 9-11, 2003. 11. Huang F. F., Sun Z. F., Shivaprasad H. L., Emerson S. U., Purcell R. H., Pierson F. W., Toth T. E., and Meng X. J. Molecular and experimental characterization of avian hepatitis E virus (avian HEV). Proceedings of the 84th Conference of Research Workers in Animal Diseases, #168. Chicago, IL, November 9-11, 2003. 12. Billam P., Huang F. F., Sun Z. F., Guenette D. K., Pierson F. W., Toth T. E., and Meng X. J. Systematic pathogenesis and replication of a strain of the hepatitis E virus (HEV) in its natural host: avian HEV infections in specific-pathogen-free adult chickens. Proceedings of the 84th Conference of Research Workers in Animal Diseases, #170. Chicago, IL, November 9-11, 2003. 13. Sun Z. F., Larsen C. T., Huang F. F., Pierson F. W., Toth T. E., and Meng X. J. Subclinical infection of avian hepatitis E virus in chicken flocks in the United States. Proceedings of the 15th Annual Research Symposium, VMRCVM, June 5-6, 2003. 169 14. Huang F. F., Haqshenas G., Sun Z. F., Pierson F. W., Toth T. E., and Meng X. J. Complete genomic sequence analyses of the newly discovered avian hepatitis E virus (avian HEV) reveals that avian HEV is genetically related to but distinct from swine and human HEVs. Proceedings of the 15th Annual Research Symposium, VMRCVM, June 56, 2003. 15. Sun Z. F., Larsen C. T., Huang F. F., Pierson F. W., Toth T. E., and Meng X. J. Experimental and field evidence of subclinical spread of avian hepatitis E virus in chicken flocks. Proceedings of the 19th Annual Graduate Student Assembly Research Symposium of Virginia Polytechnic Institute and State University, Blacksburg, March 26, 2003. 16. Sun Z. F., Larsen C. T., Huang F. F., Pierson F. W., Toth T. E., and Meng X. J. Experimental and field evidence of subclinical spread of avian hepatitis E virus in chicken flocks. Proceedings of the 83rd Conference of Research Workers in Animal Diseases, #171. St. Louis, MO. November 10-12, 2002. 17. Sun Z. F., Huang F. F., Shivaprasad H. L., Woolcock P. R., Halbur P. G., Schommer S. K., Pierson F. W., Toth T. E., and Meng X. J. Use of heteroduplex mobility assays (HMA) for pre-sequencing screening and identification of variant strains of avian and swine hepatitis E viruses. Proceedings of the 14th Annual Research Symposium, VMRCVM, June 6-7, 2002. 18. Sun Z. F., Cheung A. K., Chen Z., and McCullough D. Establishment of pseudorabies virus latency in neuroblastoma cells overexpressing the apoptosis-inhibiting Bcl-2 polypeptide. Preceedings of the 11th International Congress of Virology, Sydney, Australia, August 9-13, 1999. 19. Chen Z., Sun Z. F., Cheung A. K., and McCullough D. Induction of apoptosis in MadinDarby bovine kidney cells by pseudorabies virus. Proceedings of the 79th Conference of Research Workers in Animal Disease, Chicago, IL, November 8-10, 1998. 20. Sun Z. F., Qian Y. Q., Fang X. L., and Wen R. Neutralization and passive protection tests of monoclonal antibodies against porcine epidemic diarrhea virus. Proceedings of the 96’ Annual Conference of the Society for Animal Husbandry and Veterinary Medicine of Shanghai, Shanghai, China. January 1997. Pages 49-51. 21. Shan S. H., and Sun Z. F. Isolation and characterization of serotype I Marek’s disease virus. Proceedings of the 96’ Annual Conference of the Society for Animal Husbandry and Veterinary Medicine of Shanghai, Shanghai, China. January 1997. Pages 61-62. 22. Sun Z. F., Du N. X., and Xu W. Y. Analysis of the combining epitopes of monoclonal antibodies on rabbit hemorrhagic disease virus. Proceedings of the Annual Research Symposium, Shanghai Academy of Agricultural Sciences, Shanghai, China. November 1994. 23. Sun Z. F., Du N. X., and Xu W. Y. Detection of rabbit hemorrhagic disease virus by enzyme-linked immunosorbent assay using monoclonal antibodies. Proceedings of the 2nd National Conference of the Chinese Society for Immunology, Nanjing, China, October 1993. Page 328. 170