* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download File - Mr Francis` Weebly
Survey
Document related concepts
Transcript
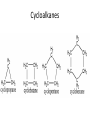
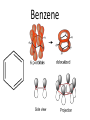
Organic Chemistry The World of Carbon Bonding • In this topic the majority of the bonding you will encounter will be covalent. • We can represent this with either an electron dot diagram or a line between atoms (-). • A hydrocarbon is a compound containing hydrogen and carbon only. • A carbohydrate contains carbon, hydrogen and oxygen. Bonding of Carbon • Carbon can bond onto itself! • Within organic chemistry carbon forms 4 bonds, this gives it a tetrahedral shape. • Whenever you are counting the bonds a carbon atom makes-look for 4! • It has 6 electrons in its outer shell arranged 1s22s2sp2 • It has room for 4 bonds to 4 other atoms. Bond angles • (A)The carbon atom forms bonds in a tetrahedral structure with a bond angle of 109.5O. • (B) Carbon-to-carbon bond angles are 109.5O, so a chain of carbon atoms makes a zigzag pattern. • (C) The unbranched chain of carbon atoms is usually simplified in a way that looks like a straight chain, but it is actually a zigzag, as shown in (B). • In effect, you draw zigzag chains Prefixes • These are the prefixes denoting the numbers involved in carbon chains (YOU MUST LEARN THESE). • Meth-1 Eth-2 Prop-3 But-4 • Pent-5 Hex-6 Hept-7 Oct-8 • Non-9 Dec-10 Hydrocarbons • The most commonly used fuels are hydrocarbons. • There are 4 main groups you are expected to know in Chemistry 2AB. • Alkanes Alkenes • Cycloalkanes Cycloalkenes • In addition to this you will learn an array of functional groups, giving you a large amount of compounds to learn to name. Alkanes • Alkanes are saturated hydrocarbons. This means that all of the bonds are single bonds. • They cannot undergo addition reactions but can take part in elimination and substitution reactions. • Alkanes are based on a hydrocarbon chains. • They all end with the suffix –ane. • General formula is CnH2n+2 Alkenes • These are unsaturated hydrocarbons. They have at least 1 double Carbon to Carbon bond which can be added to. • Can undergo addition, elimination and substitution reactions. • Suffix is –ene. If more than one –diene, -triene etc. • General formula is CnH2n but H can be less depending on number of double bonds Alkynes • Unsaturated hydrocarbon containing a triple Carbon to Carbon bond. • Same rules as alkenes. • Suffix is -yne • General formula is CnH2n-2 Cycloalkanes • Saturated hydrocarbons in which the main body is a ring structure. • Cyclo is placed as a prefix and then the compound is named like an alkane. • General Formula is CnH2n Cycloalkenes • Unsaturated hydrocarbons in which the main body is a ring structure. • Cyclo is placed as a prefix and then the compound is named like an alkene. • General Formula is CnH2n-2 Cycloalkynes • Unsaturated hydrocarbons in which the main body is a ring structure. • Cyclo is placed as a prefix and then the compound is named like an alkyne. • General Formula is CnH2n-4 Alkanes Cycloalkanes • (A)A straight-chain alkane is identified by the prefix n- for "normal" in the common naming system. • (B) A branched-chain alkane isomer is identified by the prefix iso- for "isomer" in the common naming system. In the IUPAC name, isobutane is 2methylpropane. (Carbon bonds are actually the same length.) • (A)The "straight" chain has carbon atoms that are able to rotate freely around their single bonds, sometimes linking up in a closed ring. (B) Ring compounds of the first four cycloalkanes. Aromatic Hydrocarbons • Aromatic hydrocarbons are ring structured hydrocarbons in which there are delocalised π-electrons in the ring. • π electrons are unpaired electrons in the plane of the benzene ring. • These electrons make the compound more stable than expected. • Aromatics undergo substitution reactions. Benzene Petroleum • Crude oil is a mixture of various hydrocarbons. • It is formed from the fossilised remains of marine organisms. • These organisms die and fall to the bottom of the ocean where they are covered in silt. • Over time and under extreme pressure and heat, without oxygen, they turn into crude oil. • Crude oil is processed by Fractional Distillation. Fractional Distillation Column •Crude oil is fed through a tube towards the column. •The tube is heated using a furnace. •Each fraction is separated in the column by it’s approximate boiling point. Fractions • The octane rating scale is a description of how rapidly petroleum burns. It is based on (A) n-heptane, with an assigned octane number of 0, and (B) 2,2,4trimethylpentane, with an assigned number of 100. Hydrocarbon Derivatives • Introduction – Hydrocarbon derivatives are formed when one or more hydrogen atoms is replaced by an element or a group of elements other than hydrogen. – Halogens (F2, Cl2, Br2, I2,) can all add to a hydrocarbon to form am alkyl halide. • • • • • When naming the halogen the –ine ending is replaced by –o Fluorine becomes fluoro Chlorine becomes chloro Bromine becomes bromo Iodine becomes iodo • Common examples of organic halides. – Alkenes can also add to each other in an addition reaction to form long chains of carbon compounds. • this is called polymerization – The atom or group of atoms that are added to the hydrocarbon are called functional groups. • Functional groups usually have multiple bonds or lone pairs of electrons that make them very reactive. • Alcohols – An alcohol has a hydrogen replaced by a hydroxyl (-OH) group. – The name of the hydrocarbon that was substituted determines the name of the alcohol. – The alcohol is named using the hydrocarbon name and adding the suffix –ol. • If methane is substituted with an OH group it becomes methanol • If a pentane group is substituted with an OH group it is pentanol. • For alcohols with more than two carbon atoms we need the number the chain so as to keep the alcohol group as low as possible. • Four different alcohols. The IUPAC name is given above each structural formula, and the common name is given below. • Gasoline is a mixture of hydrocarbons (C8H18 for example) that contain no atoms of oxygen. Gasohol contains ethyl alcohol, C2H5OH, which does contain oxygen. The addition of alcohol to gasoline, therefore, adds oxygen to the fuel. Since carbon monoxide forms when there is an insufficient supply of oxygen, the addition of alcohol to gasoline helps cut down on carbon monoxide emissions. An atmospheric inversion, with increased air pollution, is likely during the dates shown on the pump, so that is when the ethanol is added. – The OH group is polar and short chain alcohols are soluble in both nonpolar alkanes and water. – If an alcohol contains two OH groups it is a diol (sometimes called a glycol). – An alcohol with three OH groups is called a triol (sometimes called a glycerol). • Common examples of alcohols with one, two, and three hydroxyl groups per molecule. The IUPAC name is given above each structural formula, and the common name is given below.