* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download THE ROLE OF COMPLEMENT
Hygiene hypothesis wikipedia , lookup
Adaptive immune system wikipedia , lookup
Immune system wikipedia , lookup
Psychoneuroimmunology wikipedia , lookup
Polyclonal B cell response wikipedia , lookup
Innate immune system wikipedia , lookup
Monoclonal antibody wikipedia , lookup
Cancer immunotherapy wikipedia , lookup
Immunosuppressive drug wikipedia , lookup
Biochemical cascade wikipedia , lookup
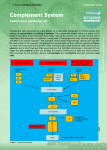
THE MULTIPLE ROLES OF COMPLEMENT Dr Andrew Guirguis Haematology Registrar The Alfred Hospital Scientific Meeting – 22nd May, 2008 History of complement Ehrlich – role of ‘complementing’ antibodies in killing of bacteria. 1895 – Bordet Subsequent discovery of components Current knowledge: – > 30 proteins in plasma + on cell surfaces – ~ 15% of globulin fraction of proteins Nomenclature C1 – C1q, C1r, C1s C4, C2, C3, C5, C6, C7, C8, C9 Many referred to as ‘zymogens’ ‘a’ and ‘b’ – added in to denote cleavage products. ‘b’ – larger fragment Alternative pathway proteins:- ‘Factors’ or identified by single letters Complement receptors:- named according to ligand (eg C6 receptor) or using CD system. The basics! ‘Innate immune system’ Cascade C3 – most important component Activation:- innate or adaptive systems – Classical:- adaptive immune system – immune complexes bind to C1q – Alternative:- innate – chance binding of C3b to microorganism surface. Distinction of self from non-self! Deficiencies:- increased susceptibility to recurrent infections (pyogenic bacteria) OR illnesses a/w production of autoantibodies + immune complexes. Main roles Defends against pyogenic bacterial infections Bridges both the innate and adaptive immunity systems Assists in disposing of immune complexes etc Role in Inflammation Opsonization:- C3b is important! 2. Chemotaxis:complement fragments diffuse from target – stimulating cellular movement and activation. 3. Target cell lysis:‘membrane attack complex hydrophobic ‘plug’ inserted into lipid membrane bilayer 1. Activation Pathways:1. Classical 2. Lectin 3. Alternative Common end point: formation of C3 convertase – cleaves to C3a and C3b – Classical + Lectin pathways – C4b2a – Alternative pathway – C3bBb Ultimately:- converted into C5 convertase – by further addition of C3b. Production of MAC. 1. Classical pathway ‘Antibody’ directed Begins with C1 C1 – Pentamolecule – C1q fragment (6 domains) + 2 x C1r + 2 x C1s – Antibody binds to two or more of the six domains (binds either IgG or IgM molecules) – C1 complex undergoes conformational change – ‘Autocatalysis’ of C1r – C1s activation 2. Lectin pathway Antibody independent – C1q – calcium-dependent lectin (collectin) – Other members:- mannan-binding lectin (MBL), conglutinin and lung surfactant A + D. – MBL – may bind mannose grps on bacterial surface – then interacts with associated Serine Proteases – MASP1 and 2 (homologous to C1r and C1s). – Antibody independent activation of classical pathway Downstream effects C1 – cleaves C4 – forming activated C4b – Two isotypes exist C4A – binding amine grps (usually on proteins) C4B – hydroxyl grps on CHO C4b – allows binding of C2. Acted on by C1s to release C2b. C4b + C2a = classical pathway convertase (C3) By definition:- C3 convertase – breaks up C3 to C3a and C3b (focus of further complement activation) What about regulation? C1 inhibitor – serine proteinase inhibitor (aka serprin) – binds and inactivates C1r and C1s Inhibition of formation of C3 convertase enzymeC4b2a (by ongoing catabolization of C4b by Factor I and C4 binding protein) Other complement control factors – inhibit complement binding to host cell surfaces – – – – DAF: (Decay accelerating factor) – CD55 CR1 MCP: Membrane co-factor protein Inhibit binding of C2 to C4b; promote ‘decay acceleration’ of C2a from C4b. Assist in catabolism of C4b by Factor I Alternative pathway Spontaneous activation – C3 is susceptible to spontaneous hydrolysis by water ‘Tick over activation’ – to form C3i C3i – acts as binding site for Factor B (cleaved by Factor D – to form Ba) C3iBb – alternative pathway C3 convertase Most C3b generated becomes inactivated in water. If it comes into contact with non-self – initiates amplification loop of alternative pathway. Regulation… it’s always about rules!!! Factor H and I DAF + CR1 – accelerate dissociation of C3bBb ‘How C3b reacts is governed by the surface to which it attaches’ – protected vs nonprotected Initiators of complement activation pathways Classical – – – – Immune complexes Apoptotic cells Viruses + GN bacteria CRP bound to ligand Lectin – Mannose groups – terminal ends of microbes Alternative – Bacteria, fungi, viruses, tumour cells etc Membrane attack complex Requires enzymatic cleavage of C5 Sequential binding of C6, C7 (hydrophobic status), C8, C9 (up to 14 monomers) Formation of lytic ‘plug’ – majority of damage caused by C9 C9 – analogous to perforin (used by T lymphocytes) C5b67 – can be inactivated by numerous means (S protein – vitronectin etc) RBC immunity: poorly lysed by homologous complement – CD59: glycophospholipid foot. Inhibits insertion + unfolding of C9 into membranes. Clinically speaking… CH50 / THC (total haemolytic complement):- requires all nine components of classical pathway to give normal value – used to screen for deficiency of classical pathway. – If very low - ? Homozygous deficiency of classical pathway component – Less dramatic reduction during inflammatory process AH50: alternative pathway measure C3/4:- helpful as activity markers in those with SLE Anaphylatoxins:- C5a / C3a – if increased – complement activation ? M’ment of split products Elevated complement levels = inflammatory response (i.e acute phase reaction) – esp C3 / C4 /B Reduced levels: often a/w disease involving immune complexes / autoantibodies. May be useful for Dx + Mx of certain diseases (eg SLE, Sjogren’s, vasculitis etc) – Low C4 / C3 + N FB – classical pathway activation – Low FB + C3 + N C4 – alternative p’way activation – C4 + FB – low = both p’ways activated Clinical implications 1. 2. 3. 4. 5. 6. 7. Complement deficiencies Glomerulonephritis C1 inhibitor deficiency SLE PNH Sepsis APLS 1. Complement deficiency:Increased susceptibility to pyogenic infections Contributing factors 1. Pyogenic infection:- – Deficient opsonisation – Deficiency compromising lytic activity – Deficient manose-binding lectin pathway Site of defect:- antibody production, complement proteins of classical pathway, phagocyte function Usually bacteria is opsonised with Ab – complement is then activated, phagocytosis occurs and intracellular killing Key player:- C3b MAC component deficiency – a/w Neisserial disease* Risk of meningococcal disease ~ 0.5% / yr (RR 5000 cf normal population) Deficiency occurs due to 1 of 3 point mutations – a/w reduced levels. Associated with higher risk of infection in children – whilst losing passive immunity ? Protective against mycobacterial infections 2. 3. Impaired lysis Deficient lectin 2. Glomerulonephritis… Key of C3b regulation:- whether Factor B or H binds to C3b If C3 regulation is defective:- often a/w GN. – Due to C3 nephritic factor – increases stability of C3 convertase enzymes – association with membranoproliferative GN OR – Reduced function of Factor H or I ? Associations with HUS (+/- low level of C3) 3. C1 INHIBITOR DEFICIENCY Autosomal dominant – inadequate production of physiologically adequate C1 inhibitor – Type 1:- 85% - reduced transcription of abnormal allele. Reduced levels of C1 inhibitor – Type 2:- point mutation in C1 inhibitor gene – altered activity (So levels may be normal or high – as not consumed) – Autoantibodies against C1 inhibitor Inhibits – C1r and C1s, activated FXI and XII Consumed by plasmin – trigger for angioedema attacks. Rx: C1 inhibitor infusion. 4. Complement deficiency + SLE Inverse correlation with position of deficient protein in activation sequence of the classical pathway – Homozygous def of C1q, C1r and C1s + C4 – strongly a/w SLE (93%, 57%, 75%) – Cf. def of C2 – 10% prevalence. Protective role exists for those in whom activation of classical pathway up to C4 cleavage occurs. 5. PNH:Acquired stem cell disorder Deficiency of PIG-A (somatic mutation) – required for synthesis of glycosyl-PI phospholipid. Important for anchorage of proteins to cell membranes In PNH – lack of GPI-linked proteins (including complementregulating surface proteins) - eg DAF (i.e CD55) which regulates formation of C3 convertase and CD 59 – restricts formation of MAC. – Deficiency on RBCs:- does not allow protection against terminal complement Clinically: chronic haemolysis, fatigue, pain, thrombotic events. median age – early 30s; median survival as low as 1015 yrs. Smooth muscle dystonia - ? 2’ to NO depletion during chronic haemolysis Who to screen? Hb’uria Coombs –ve haemolytic anaemia Those with AA or MDS (annual screen) Haemolytic anaemia VT without explanation (including unusual sites – eg mesenteric, portal, cerebral etc) Unexplained arterial thrombosis Episodic dysphagia or abdo pain Parker et al, 2005 Dx: Flow cytometry: gold standard (peripheral blood). Granulocytes provide best estimate of PNH clone size. Role of Soliris (eculizumab) Other Rx:-Supportive transfusions - Haematinic supplementation - Anticoagulation (for those with Hx of thrombosis or for prophylaxis) -Therefore multiple benefits - Risks?? 6. Complement system + sepsis C5a – anaphylatoxin – strong chemoattractant. Sepsis – excessive early production of C5a – upregulated proinflammatory response. ? Role for blockade of C5a with antibodies – shown to improve survival of septic mice. ? Use in IHD to assist cardiac reperfusion 7. Complement + APLS Nature medicine 2004:– Previous mouse models – shown that complement activation plays an important role in pregnancy + fetal growth restriction – Likely induced by activation thru aPL antibodies (classical pathway) – Anticoagulation alone – insufficient in completely averting miscarriage – Heparin use - ? Additional role via inhibition of complement. APLS Mouse model used:– Pregnant mice injected with aPL antibodies – Rx:- heparin (UFH or LMWH) – reduced frequency of fetal resorption to that of healthy controls. – To rule out mere ‘anticoagulant effect’ – use of fondaparinux or hirudin – both do not directly affect the complement systems. In vivo:– Focus on C3 and degradation products – increased levels seen with aPL-IgG injection. – Abolished by UFH or LMWH, but not by fondaparinux or hirudin. Separate study:- use of Crry-Ig (complement receptor 1related gene / protein y)(exogenous inhibitor of C3 activation) OR C3 deficient mice – similar results. – Associated with fewer resorptions and less antibody-mediated growth retardation Activation of complement – associated with thrombophilic state A role for ‘complement’ary medicine??