* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Carboxylic Acids - BSAK Chemistry weebly
Artificial photosynthesis wikipedia , lookup
Hydrogen bond wikipedia , lookup
Peptide synthesis wikipedia , lookup
Citric acid cycle wikipedia , lookup
Transition state theory wikipedia , lookup
Atomic theory wikipedia , lookup
Water splitting wikipedia , lookup
Physical organic chemistry wikipedia , lookup
Liquid–liquid extraction wikipedia , lookup
Flux (metallurgy) wikipedia , lookup
Asymmetric induction wikipedia , lookup
Hypervalent molecule wikipedia , lookup
Acid dissociation constant wikipedia , lookup
Stoichiometry wikipedia , lookup
Electrochemistry wikipedia , lookup
Bioorthogonal chemistry wikipedia , lookup
Chemical reaction wikipedia , lookup
Biosynthesis wikipedia , lookup
Electrolysis of water wikipedia , lookup
Click chemistry wikipedia , lookup
Hydroformylation wikipedia , lookup
Stille reaction wikipedia , lookup
Acid strength wikipedia , lookup
Biochemistry wikipedia , lookup
Hydrochloric acid wikipedia , lookup
Strychnine total synthesis wikipedia , lookup
Metalloprotein wikipedia , lookup
Hydrogen-bond catalysis wikipedia , lookup
Petasis reaction wikipedia , lookup
Acid–base reaction wikipedia , lookup
Lewis acid catalysis wikipedia , lookup
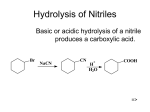
Carboxylic Acids Naming: carbon chain stem + oic General Information • Functional group carboxyl Examples Ethanoic Benzoic Physical Properties The boiling point of propane is -42oC Q: Will the b.p. of propan-1-ol be lower or higher than that of propane? Explain. A: Significantly higher, due to hydrogen bonding. propan-1-ol CH3CH2CH2OH 97.2°C Q: What about the b.p. of ethanoic acid? A: Higher still due to the formation of dimers. ethanoic acid CH3COOH 118°C Q: Are carboxylic acids soluble in water? Explain. A: The polar C=O and the ionic O- group mean that short chain (up to C4) carboxylic acids are readily water soluble due to H bond formation with water molecules. Q: With larger chain acids solubility decreases proportionally with size. Why? A: Hydrocarbon chains are hydrophobic (water fearing). Chemical Properties Weak acids - partially dissociate in water: Are carboxylic acids more or less acidic than alcohols? Explain Acidity The OH group in the acids is more acidic than the OH group in the alcohols The carboxylate ion (COO-) is stabilised by delocalisation of electrons (pi orbitals) Q: How are they made? A: Oxidation of primary alcohols and aldehydes OR : Hydrolysis of a nitrile by boiling with dilute acid Aromatic Carboxylic Acids • Benzoic acid is produced by oxidation of the side chain Q: What test would you use for the carboxylic acid functional group? A: Add PCl5 - misty white fumes of HCl are produced and this confirms the presence of the OH group. It also produces a second product called an acyl chloride. CH3COOH + PCl5 CH3COCl + HCl + POCl3 Reactions of Carboxylic Acids Looking at the structures of the carboxylic acids, what type of reactions will they undergo? In your groups write your ideas on a piece of paper. Don’t forget to justify them! 3 main sites of reaction • -OH bond gives rise to acidity • -C=O bond undergoes nucleophilic addition reactions • -C-O bond undergoes substitution reactions Formation of Esters • General reaction from acid and alcohol as learnt at AS: • At A2 we need to see how esters can be formed from acyl chlorides Formation of Acyl Chlorides • PCl5 generates the nucleophile Cld-which replaces the OH group in a substitution reaction: CH3COOH + PCl5 CH3COCl + HCl + POCl3 What other reagents can be used to generate an acyl chloride from a carboxylic acid? Write equations for ethanoic acid: Acyl Chlorides • • • • • Derivatives of carboxylic acids Contain an acyl group Reactive substances Intermediates in organic synthesis Also called acid chlorides Naming Acyl Chlorides carboxylic acid name ethanoic acid acyl chloride name ethanoyl chloride acyl chloride formula CH3COCl propanoic acid propanoyl chloride CH3CH2COCl butanoic acid butanoyl chloride CH3CH2CH2COCl Properties of Acyl Chlorides • Colourless fuming liquids • Strong smell - a mixture of the smell of vinegar (ethanoic acid) and the acrid smell of hydrogen chloride gas. • The smell and the fumes are because ethanoyl chloride reacts with water vapour in the air. Reactions of Acyl Chlorides Ethanoyl chloride reacts vigorously with water (Hydrolysis) Predict what the products of this reaction might be: Mechanism Q: What type of reaction is this? In your groups predict a mechanism for the reaction. A: Nucleophilic addition/elimination Q: In your groups draw the mechanism for this reaction. Clue: There are three stages • The first stage (the addition stage of the reaction) involves a nucleophilic attack on the fairly positive carbon atom by one of the lone pairs on the oxygen of a water molecule. • The second stage (the elimination stage) happens in two steps. In the first, the carbonoxygen double bond reforms and a chloride ion is pushed off. • This is followed by removal of a hydrogen ion by the chloride ion to give ethanoic acid and hydrogen chloride Ease of hydrolysis Predict and explain the order of relative ease of hydrolysis of the following: • Acyl chlorides • Alkyl chlorides • Aryl chlorides You should include diagrams and equations where appropriate • Acyl chlorides: These contain a -COCl group, e.g. ethanoyl chloride, CH3COCl, or benzoyl chloride, C6H5COCl • Alkyl chlorides: These have a chlorine attached to a carbon chain, e.g. chloroethane, C2H5Cl • Aryl chlorides: These have a chlorine attached directly to a benzene ring, e.g. chlorobenzene, C6H5Cl Answer Acyl chlorides are much more reactive towards hydrolysis than alkyl chlorides. Aryl chlorides are resistant to hydrolysis. Why? Notice that the carbon atom being attacked by a nucleophile is made more positive by the fact that there are two electronegative atoms attached to it. In an alkyl chloride, all you have attached is one chlorine atom which is fairly, but not very, electronegative. Esters from Acyl Chlorides • If you add an acyl chloride to an alcohol, you get a vigorous (even violent) reaction at room temperature producing an ester and misty fumes of hydrogen chloride. • Add the liquid ethanoyl chloride to ethanol, you get a burst of hydrogen chloride produced together with the liquid ester ethyl ethanoate. In your groups • What reactants are required to make phenyl ethanoate? • Write an equation to show this reaction: Benzoyl Chloride • Benzoyl chloride has the formula C6H5COCl. • How does the reactivity of benzoyl chloride compare to that of ethanoyl chloride? Explain. • The -COCl group is attached directly to a benzene ring. It is much less reactive than simple acyl chlorides like ethanoyl chloride. Acyl chlorides with ammonia • Ethanoyl chloride reacts violently with a cold concentrated solution of ammonia. • A white product is formed which is a mixture of two solid products Predict what the products will be and write an equation for this reaction. • The name of the major product is ethanamide • Unlike the reactions between ethanoyl chloride and water or ethanol, hydrogen chloride isn't produced – why not? Formation of ethanamide • In your groups draw out the mechanism for this reaction • Explain why 2 moles of ammonia are needed for this reaction • The first stage (the addition stage of the reaction) involves a nucleophilic attack on the fairly positive carbon atom by the lone pair on the nitrogen atom in the ammonia. • The second stage (the elimination stage) happens in two steps. In the first, the carbonoxygen double bond reforms and a chloride ion is pushed off. • That is followed by removal of a hydrogen ion from the nitrogen. This might happen in one of two ways: • It might be removed by a chloride ion, producing HCl (which would immediately react with excess ammonia to give ammonium chloride as above) . . . • And… • . . or it might be removed directly by an ammonia molecule • The ammonium ion, together with the chloride ion already there, makes up the ammonium chloride formed in the reaction. Reactions with amines • Ethanoyl chloride reacts violently with a cold concentrated solution of ethylamine. A white solid product is formed which is a mixture of N-ethylethanamide (an N-substituted amide) and ethylammonium chloride. N means that the methyl group is attached to the nitrogen atom of the amide.