* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Angular Momentum Quantization
Atomic nucleus wikipedia , lookup
Quantum electrodynamics wikipedia , lookup
Identical particles wikipedia , lookup
Compact Muon Solenoid wikipedia , lookup
Canonical quantization wikipedia , lookup
Quantum vacuum thruster wikipedia , lookup
Standard Model wikipedia , lookup
Bell's theorem wikipedia , lookup
Quantum state wikipedia , lookup
Uncertainty principle wikipedia , lookup
Elementary particle wikipedia , lookup
Electron scattering wikipedia , lookup
Tensor operator wikipedia , lookup
Introduction to quantum mechanics wikipedia , lookup
Old quantum theory wikipedia , lookup
Relativistic quantum mechanics wikipedia , lookup
Spin (physics) wikipedia , lookup
Theoretical and experimental justification for the Schrödinger equation wikipedia , lookup
Symmetry in quantum mechanics wikipedia , lookup
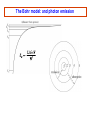
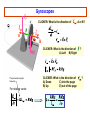
PHYS 172H: Modern Mechanics Fall 2010 Lecture 20 – Angular Momentum Quantization (mostly) Chapter 11.8 – 11.11 CLICKER POLL Not for Credit How many senses does the human body have? (Taste, smell...) A) 3 senses B) 4 senses C) 5 senses D) 6 senses E) More than 6 Predicting Position with Rotation A light string is wrapped around disk of radius R and moment of intertia I that can freely spin around its fixed axis. The string is pulled with force F during time Δt. Assume that the disk was initially at rest (ωi=0) 1) What will be the angular speed ωf ? I θ R m F=mg Solution: Predicting Position with Rotation I θ A light string is wrapped around disk of radius R and moment of inertia I that can freely spin around its fixed axis. The string is pulled with force F during time Δt. Assume that the disk was initially at rest (ωi=0) 1) What will be the angular speed ωf ? 2) How far (Δx) will the end of string move? Solution: R m F=mg ω changes linearly with time: This is the rotational analog of accelerated linear displacement See also examples in Chapter 11.8 And this string motion is in the linear “world”, once we multiply the angular displacement by R Angular momentum quantization Many elementary particles behave as if they posses intrinsic rotational angular momentum Electron can have translational (orbital around nucleus), and intrinsic rotational angular momenta Strange but true: Angular momentum is quantized Angular momentum quantum = Whenever you measure a vector component of angular momentum you get either half-integer or integer multiple of Orbital angular momentum comes in integer multiples, but intrinsic spin of “Fermions” (building blocks) is ½ unit of Orbital Angular Momentum Where is the orbital angular momentum in a hydrogen orbital? + px i py Electron "current" circles around the atom. = |L=1, Lz=1> Quantized because these are 3D standing electron waves around the nucleus. See Atom in a Box www.daugerresearch.com Bohr’s Atomic Model LA,trans ,electron = mrkqe2 Niels Bohr 1913: IDEA: Electron can only take orbits where its translational angular momentum is integer multiple of Allowed radii: 2 r = N2 2 kqe me = 1.05 ×10−34 J ⋅ s N = 1, 2,3,… This implies that only certain values of LA,trans,electron are allowed: LA,trans ,electron = N where N=1,2,3,… NOTE: Because K and U are functions of r and v, energy levels are quantized also. Bohr Model Consider an electron in circular orbit A about a proton. What are the possible values of LA,trans,electron? Assume circular motion: pv Fe = r Thus, ⇒ kqe2 mv 2 = 2 r r kqe2 ⇒ v= mr LA,trans ,electron = mvr = mrkqe2 If any orbital radius r is allowed, LA,trans,electron can be anything. However, only certain values of r are allowed . . . The Bohr model: allowed radii and energies See derivation on page 444-446 Allowed Bohr radii for electron orbits: This is 2K Use EN = K+U and Bohr model energy levels: The Bohr model: and photon emission Gyroscopic Stability Edmund Scientifics In 1917, the Chandler Company of Indianapolis, Indiana, created the "Chandler gyroscope,” a toy gyroscope with a pull string and pedestal. It has been in continuous production ever since and is considered a classic American toy. -- Wikipedia Best Trick in the Book Vectors have direction and magnitude. Vector Notation and the Momentum Principle: Use the chain rule causes changes in the direction of causes changes in the magnitude of Best Trick Not in the Book Vectors have direction and magnitude. Vector Notation and the Angular Momentum Principle: Use the chain rule causes changes in the direction of causes changes in the magnitude of Gyroscopes Precession Precession and nutation As you saw on Wednesday. Look straight down on the model at the left: the Torque and the Angular Impulse are COUNTERCLOCKWISE, and indeed that’s how the gyroscope precesses here. Note that the angular impulse, being perpendicular to L, STEERS L but does not increase or decrease L. To understand better, it’s useful to note: this is somewhat analogous to centripetal force STEERING momentum, in circular motion, without speeding or slowing the tangential velocity. Gyroscopes M CLICKER: What is the direction of , A or B? A B N CLICKER: What is the direction of A) Left B) Right The precession angular frequency For rotating vector: CLICKER: What is the direction of A) Down C) into the page B) Up D) out of the page ? ? i>clicker NOTE: if the shaft of the gyro isn’t horizontal, R must be replaced by Rcos(θ) where θ is the shaft’s angle above horizontal and is the complement of the angle between R and g. Fast precession A Slow precession B In which of the two gyroscopes does the disk spin faster? Precession phenomena (see book) Magnetic Resonance Imaging (MRI) Precession of spin axes in astronomy (moon pulling on Earth’s bulging “midriff” as mentioned last time) Tidal torques NMR - nuclear magnetic resonance Independently discovered (1946) Nobel Prize (1952) Felix Bloch Edward Mills Purcell 1912-1997 1905-1983 B.S.E.E. from Purdue electrical engineering V. Barnes had the good luck to take introductory E&M from Prof. Purcell as an undergrad at Harvard. Particle spin angular momentum Electron, muon, neutrino have spin 1/2 : measurements of any component of their angular momentum yields ±½ħ (along any chosen axis.) Quarks have spin ½ Protons and neutrons (three quarks) have spin ½ Fermions: spin ½ are ”matter particles” or “building blocks” They obey the Pauli exclusion principle Two identical Fermions cannot be in the same quantum state in the same place Two lowest energy electrons in any atom have orbital angular momentum 0 One has spin up, the other has spin down (+- ½ along any chosen axis) The different spin projections distinguish between the two electrons so that they are not “identical” for Pauli exclusion purposes. The two spin projections add up to 0, and when added to the above 0 orbital, the TOTAL ANGULAR MOMENTUM is 0 Particle spin angular momentum Bosons: integer spin = Force carrying particles Mesons: (quark+antiquark) have spin 0 or 1: composite, “old” nuclear force Gluons (elementary, the “new” strong color force) photons, gravitons, weak bosons W and Z All have spin 1. These are the force carriers of Quantum Field Theory Higgs particle (if it exists) has spin 0 Particle spin Rotational angular momentum Macroscopic objects: quantization of L is too small to notice! Rotational energies of molecules are quantized Quantum mechanics: Lx, Ly, Lz can only be integer or half-integer multiple of ħ Quantized values of where l is integer or half-integer These rules are not at all intuitive. Rotation is a richly complicated phenomenon (as you have been told in lectures), and even more-so in the Quantum world. But the quantization of the PROJECTION of angular momentum along any chosen axis plays very directly into the structure of the microworld that we hope you get a sense of in this course.