* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Why chemokines?
Immune system wikipedia , lookup
Lymphopoiesis wikipedia , lookup
Molecular mimicry wikipedia , lookup
Adaptive immune system wikipedia , lookup
Cancer immunotherapy wikipedia , lookup
Polyclonal B cell response wikipedia , lookup
Psychoneuroimmunology wikipedia , lookup
Immunosuppressive drug wikipedia , lookup
Innate immune system wikipedia , lookup
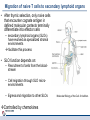
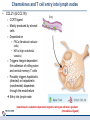
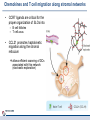
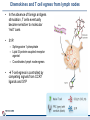
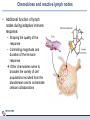
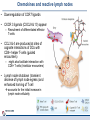
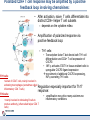
Orchestrating the orchestrators: chemokines in control of T cell traffic December 20th 2010 Kathrin Hüging Why chemokines? • Chemokines – are small chemoattractant proteins – stimulate the migration and activation of cells, especially phagocytic cells and lymphocytes – are secreted molecules, • in vivo they are “seen“ by leukocytes while bound to extracellular matrix molecules or to cell surfaces via proteoglycans – serve a central function in the immune system by coordinating the localization of immune cells in the body to generate an immune response at specific anatomic sites – Regulate cell motility and cell adhesion through ligation of G protein-coupled receptors expressed on leukocytes Migration of naive T cells to secondary lymphoid organs • After thymic selection, only naive cells that encounter cognate antigen in defined molecular contexts terminally differentiate into effector cells – secondary lymphoid organs (SLOs) have evolved as specialized stromal environments facilitate this process • SLO function depends on: – Recruitment of cells from the bloodstream – Cell migration through SLO microenvironments – Egress and migration to other SLOs Controlled by chemokines Molecular Biology of the Cell. 3rd edition. Chemokines and T cell entry into lymph nodes • CCL21 (& CCL19): – CCR7 ligand – Mainly produced by stromal cells – Deposited on • FRCs (fibroblastic reticular cells) • HEVs (high endothelial venules) – Triggers integrin-dependent firm adhesion of rolling naive and central memory T cells – Possibly triggers haptotactic (directed) or hatpokinetic (nondirected) diapedesis through the endothelium Entry into lymph node haptokinesis= substrate dependent migration along an adhesion gradient (immobilized ligand) Chemokines and T cell migration along stromal networks • CCR7 ligands are critical for the proper organization of SLOs into – B cell follicles – T cell areas • CCL21 promotes haptokinetic migration along the stromal reticulum allows efficient scanning of DCs associated with this network (stochastic exploration) Chemokines and T cell egress from lymph nodes • In the absence of foreign antigens stimulation, T cells eventually become sensitive to molecular “exit“ cues • S1P: – Sphingosine 1-phosphate – Lipid G protein-coupled receptor agonist – Coordinates lymph node egress • T-cell egress is controlled by competing signals from CCR7 ligands and S1P Chemokines and reactive lymph nodes • Additional function of lymph nodes during adaptive immune response: – Shaping the quality of the response – Controlling magnitude and duration of the immune response Other chemokines serve to broaden the variety of cell populations recruited from the bloodstream and to orchestrate cellular collaborations Chemokines and reactive lymph nodes • Downregulation of CCR7 ligands • CXCR 3 ligands (CXCL9 & 10) appear – Recruitment of differentiated effector T-cells • CCL3 & 4 are produced at sites of cognate interactions of DCs with CD4+ helper T-cells (guided encounters) – might also facilitate interaction with CD8+ T cells (tricellular encounters) • Lymph node shutdown (transient decrese of lymph node egress) and enhanced homing of T cell accounts for the initial increase in lymph node cellularity Chemokine guidance into peripheral tissue • Naive lymphocytes circulate mainly among SLOs • Antigen-experienced lymphocytes upregulate various combinations of adhesion, chemokine and lipid chemoattractant receptors – Specific combination allows T cells to interact with blood vessel endothelium and to migrate into and within distinct peripheral tissues – e.g. CCR9: small intestine; CCR4 & CCR10: skin surveillance and response to inflammatory stimuli • Lymphocyte trafficking may become less restricted in conditions of inflammation tissue-specific vs. inflammation-induced chemokine and adhesion receptors Innate NKT and γδ T cells • During an immune response, distinct T cell subsets are generated in the lymphoid compartment • Chemokines guide these subsets into sites of inflammation and infection • Before the generation of antigen-specific T cell subsets, NKT and γδ T cells ( „innate T cells“) provide the first line of host defense • Are also guided to sites of inflammation and infection by chemokines • Receptor expression patterns suggests different trafficking potentials Chemokine receptors on innate NKT and γδ T cells Chemokine receptors on T cell subsets Polarized CD4+ T cell response may be amplified by a positive feedback loop involving chemokines: • After activation, naive T cells differentiate into distinct CD4+ helper T cell subsets – depends on the cytokine milieu • Amplification of polarized response via positive feedback loop: – TH1 cells: • Transcription factor T-bet directs both TH1 cell differentiation and CD4+ T cell expression of CXCR3 • INF-γ activates STAT1 in tissue-resident cells to upregulate CXCR3 ligand expression recruitment of additional CXCR3-expressing, INF-γ-secreting TH1 cells •TH1 cells: •subset of CD4 T cells, mainly involved in activating macrophages (sometimes called • inflammatory CD4 T cells) •TH2 cells: Regulation especially important for Th17 response: • mainly involved in stimulating B cells to produce antibody, (often called helper CD4 T cells) • amplification may drive many autoimmune inflammatory conditions Control of the feedback loop by regulatory T cells • Depending on the cytokine milieu in which the Treg cells are activated, these cells might express distinct chemokine receptor expression profiles – Overlap of chemokine receptor expression profile of Treg cells and diverse effector T cell subsets Should allow Treg cells to localize together with diverse effector T cells to control the immune response • Better understanding of this mechanism should allow therapeutic targeting to treat inflammatory conditions Conclusion • Chemokine system orchestrates T cell migratory patterns to generate, deliver and regulate specific types of immune responses in specific anatomic microenvironments • Controls T cell trafficking in the lymphoid compartment and in peripheral tissue • To allow therapeutic interference with this system, a deeper understanding of chemokine function is needed: • How do chemokines coordinate the encounters between different immune cells for information exchange? • How is specificity achieved despite so much redundancy in the chemokine system? • What is the importance of chemokines in cellular functions other than migration? Questions? Have a save trip home & a nice vacation!