* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download File
Molecular evolution wikipedia , lookup
Community fingerprinting wikipedia , lookup
Genome evolution wikipedia , lookup
Gene expression wikipedia , lookup
Secreted frizzled-related protein 1 wikipedia , lookup
Genetic engineering wikipedia , lookup
Promoter (genetics) wikipedia , lookup
Expression vector wikipedia , lookup
Gene therapy of the human retina wikipedia , lookup
Gene expression profiling wikipedia , lookup
Vectors in gene therapy wikipedia , lookup
Gene regulatory network wikipedia , lookup
Silencer (genetics) wikipedia , lookup
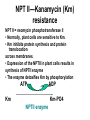
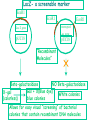
Unit III Lecture 2 B. Tech. (Biotechnology) III Year V th Semester EBT-501, Genetic Engineering Unit III • Gene library: Construction cDNA library and genomic library, Screening of gene libraries – screening by DNA hybridization, immunological assay and protein activity • Marker genes: Selectable markers and Screenable markers, nonantibiotic markers, • Gene expression in prokaryotes: Tissue specific promoter, wound inducible promoters, Strong and regulatable promoters; Increasing protein production; • Fusion proteins; Translation expression vectors; DNA integration into bacterial genome; Increasing secretions; Metabolic load, • Recombinant protein production in yeast: Saccharomyces cerevisiae expression systems • Mammalian cell expression vectors: Selectable markers; Selectable and screenable markers A marker gene is used in molecular biology to determine if a piece of DNA has been successfully inserted into the host organism. There are two types of marker genes: • Selectable markers • Antibiotic marker • Non-Antibiotic marker • Endogenous marker • Nutritional markers • Screenable markers. • Flourescence marker e.g. GFP, RFP • Colorimetric assay e.g. GUS, Lac Z • Selectable markers are required for the maintenance of the plasmid in the cell. • Under the selective conditions only, the cells that contain plasmids with the appropriate selectable marker are able to survive. • Commonly, genes that confer resistance to various antibiotics (antibotics markers) are used as selective markers in cloning vectors. • But at times nutritional markers or endogenous markers are used as selectable markers. The drawbacks of selectable markers • loss of selective pressure as a result of antibiotics degradation and inactivation. • contamination of the product or biomass by antibiotics, which may be unacceptable from medical or regulatory considerations. Selectable markers A selectable marker will protect the organism from a selective agent that would normally kill it or prevent its growth. In most applications, only one in a several million or billion cells will take up DNA. Rather than checking every single cell, scientists use a selective agent to kill all cells that do not contain the foreign DNA, leaving only the desired ones. • Antibiotics are the most common selective agents. In bacteria, antibiotics are used almost exclusively. In plants, antibiotics that kill the chloroplast are oftenused as well, although tolerance to salts and growth-inhibiting hormones is becoming more popular. Screenable markers • Reporter genes are also known as screenable marker genes. • These differ from selectable marker genes in that they do not confer a property that allows transformed cells to survive under selective conditions. • Instead, screenable markers encode a product that can be detected using a simple and inexpensive assay. • When controlled by a strong constitutive promoter, reporter genes are often used as markers to confirm transient or stable transformation, since only cells containing the reporter-gene construct can express the corresponding protein • Importantly, the assays used to detect reporter-gene activity are quantitative, so they can also be used to measure transformation efficiency. • If attached to a cloned promoter, reporter genes can therefore be used to determine transcriptional activity in different cell types and under different conditions. • Transient reporter assays have been widely used to characterize and dissect the regulatory elements driving eukaryotic genes, as shown in the example below. • The use of reporters is advantageous, because it circumvents the necessity to derive different assays for individual genes and also allows the activities of transgenes and homologous endogenous genes to be distinguished in the same cell. An example of in vitro promoter analysis using chloramphenicol acetyltransferase • The first reporter gene to be used in animal cells was cat, derived from E. coli transposon Tn9. • This gene encodes the enzyme chloramphenicol acetyltransferase (CAT), which confers resistance to the antibiotic chloramphenicol by transferring acetyl groups on to the chloramphenicol molecule from acetyl-CoA. Commonly use Screenable markers • A marker for screening will make cells containing the gene look different. There are three types of screening commonly used: • Green fluorescent protein (GFP) makes cells glow green under UV light. A specialized microscope is required to see individual cells. Yellow and red versions are also available, so scientists can look at multiple genes at once. It is commonly used to measure gene expression. • GUS assay (using β-glucuronidase) is an excellent method for detecting a single cell by staining it blue without using any complicated equipment. The drawback is that the cells are killed in the process. It is particularly common in plant science. • Blue/white screening is used in bacteria. The lacZ gene makes cells turn blue in special media (e.g. X-gal). A colony of cells with the gene can be seen with the naked eye. Markers for Screening GUS: betaglucuronidase in plants 5-bromo-4-chloro-indolyl glucuronide ( -gluc): 4-methyl-umbelliferyl-beta-D-glucuronide ( Beta-glucuronidase 37C + ( -gluc) + ( -gluc) The GUS histochemical staining assay O2 + + + gluc Hydrolysis of the X-Gluc substrate by the GUS enzy Beta-glucuronidase -gluc) Dimerization of the Gluc product by reaction with O2 37C + + gluc Fluorogenic staining Hydrolysis of the X-Gluc substrate by the GUS Fluorescent of the released enzyme fluorescent product Endogenous Markers in Animal cells Selectable markers allows differential multiplication of only transfected animal cells are utilized to select the transfected cells E.g. thymidine kinase TK gene whnen used with TK- (TK minus) cells Screenable markers or Reporters or scorable markers produces a phenotype which helps in detection the expression or level of transcripts of trans gene or recombinant gene Are used to studying promoter/enhancer activity E.g. CAT gene from E.coli transposone Tn9 Beta galactosidase gene from E.coli Luciferase gene from bacteria or firefly (Photinus pyralis) de novo and salvage nucleotide synthesis pathways De novo purine nucleotide synthesis (shown on the right of next slide) initially involves the formation of inosine monophosphate (IMP) which is then converted into either adenosine monophosphate (AMP) or, via xanthine monophosphate (XMP), guanosine monophosphate (GMP). The de novo synthesis of IMP requires the enzyme dihydrofolate reductase (DHFR), whose activity can be blocked by aminopterin or methotrexate. In the presence of such inhibitors, cell survival depends on nucleotide salvage, as shown on the left. Cells lacking one of the essential salvage enzymes, such as HPRT or APRT, therefore cannot survive in the presence of aminopterin or methotrexate unless they are transformed with a functional copy of the corresponding gene. Thus, the genes encoding salvage-pathway enzymes can be used as selectable markers. Note that the enzyme XGPTR, which converts xanthine to XMP, is found only in bacterial cells and not de novo and salvage nucleotide synthesis pathways NPT II—Kanamycin (Km) resistance NPT II = neomycin phosphotransferase II • Normally, plant cells are sensitive to Km. • Km inhibits protein synthesis and protein translocation across membranes. • Expression of the NPTII in plant cells results in synthesis of NPTII enzyme • The enzyme detoxifies Km by phosphorylation ATP Km ADP Km-PO4 NPTII enzyme LacZ- a screenable marker EcoR1 EcoR1 EcoR1 Interrupted Lac Z gene Lac gene pUC18 pUC18 “Recombinant Molecules” Beta-galactosidase NO Beta-galactosidase Gal + X(Blue dye) X-gal White colonies blue colonies (colorless) Allows for easy visual “screening” of bacterial colonies that contain recombinant DNA molecules