* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download (1)
Discovery and development of proton pump inhibitors wikipedia , lookup
Discovery and development of direct thrombin inhibitors wikipedia , lookup
Discovery and development of integrase inhibitors wikipedia , lookup
Pharmacokinetics wikipedia , lookup
Discovery and development of cephalosporins wikipedia , lookup
Specialty drugs in the United States wikipedia , lookup
Polysubstance dependence wikipedia , lookup
Discovery and development of non-nucleoside reverse-transcriptase inhibitors wikipedia , lookup
Drug discovery wikipedia , lookup
Orphan drug wikipedia , lookup
Psychedelic therapy wikipedia , lookup
Neuropharmacology wikipedia , lookup
Pharmacognosy wikipedia , lookup
Pharmaceutical industry wikipedia , lookup
Prescription costs wikipedia , lookup
Prescription drug prices in the United States wikipedia , lookup
Pharmacogenomics wikipedia , lookup
Psychopharmacology wikipedia , lookup
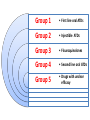
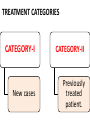
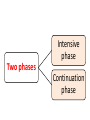
Anti-tubercular drugs Prof. Anuradha Nischal • Deadly infectious disease caused by MYCOBACTERIUM TUBERCULOSIS • Affects the lungs but can also affect other parts of the body. Epidemiology It is currently estimated that 1/2 of the world's population (3.5 billion) is infected with Mycobacterium tuberculosis. Transmission • Pulmonary tuberculosis • Droplets • Patients with the active disease (bacilli) expel them into the air by: – coughing – sneezing Signs & symptoms • A bad cough that lasts 3 weeks or longer • Coughing up blood or sputum (phlegm from deep inside the lungs) • Fever • Weakness or fatigue • Weight loss • Anorexia • mailaise • Pain in the chest Group 1 • First line oral ATDs Group 2 • Injectible ATDs Group 3 • Flouroquinolones Group 4 • Second line oral ATDs Group 5 • Drugs with unclear efficacy Group 1/First line drugs include: ISONIAZID RIFAMPICIN PYRAZINAMIDE ETHAMBUTOL most potent and best tolerated oral drugs HIGH EFFICACY AND LOW TOXICITY Group 2/Injectable ATDs Streptomycin Kanamycin Amikacin Capreomycin potent, bactericidal, but injectable drugs Group 3/Flouroquinolones Ofloxacin Levofloxacin Moxifloxacin Ciprofloxacin(resistance) so removed potent bactericidal well tolerated oral drugs. MDR Group 4/Second line oral drugs include: ETHIONAMIDE PROTHIONAMIDE CYCLOSERINE TERIZODONE p-AMINOSALICYLIC ACID less effective, bacteriostatic, more toxic drugs for resistant tuberculosis Group 5/Drugs with unclear efficacy Thiacetazone Clarithromycin Clofazimine Linezolid Amoxicillin/clavulanate Imipenem/cilastin Drugs with uncertain efficacy, may be used for XDR ISONIAZID[H] Cheapest ATD Mycobactericidal Bactericidal for rapidly growing bacilli Quiscent ones are only inhibited Extra and intracellular bacilli Equally active in acidic & alkaline medium. MOA • Inhibits mycolic acids synthesis (unique component MBCW) • High selectivity for mycobacteria MOA of INH: ISONIAZID Reactive metabolite Kat G( catalase peroxidase in mycobacteria) Inh A & Kas A Inh DHFR Inhibits the synthesis of Mycolic Acid Inh DNA syn Mechanism of Resistance • High level resistance is due to mutation in catalase peroxidase (Kat G) gene Resistance may also develop due to mutation in Kas A & Inh A gene • Efflux Of INH • Loss of INH concentrating ability of bacteria • Absorption: completely absorbed orally • Distribution: penetrate all body tissue tubercular cavities, placenta & meninges • Metabolism: in liver • Excretion: in urine • C/I known hypersensitivity acute hepatic disease Peripheral Neuropathy And neurological manifestations Paresthesias, numbness, mental disturbances most important dose dependent toxic effects. • Pyridoxine deficiency • Interference with activation of pyridoxine and its increased excretion in urine • Q- Why Vitamin B6 is given with INH Pyridoxine deficiency Interference with activation of pyridoxine and its increased excretion in urine • Pyridoxine given prophylactically (10mg/day) prevents neurotoxicity • INH neurotoxicity is treated by pyridoxine 100mg/day WHOM Must: Diabetics, Chr. Alcoholics, malnourished, pregnant, lactating & HIV infected patients Hepatitis Common in older adults & alcoholics Dose related damage to hepatic cells reversible RIFAMPICIN[R] • Semi synthetic derivative of Rifamycin B from St. meditarranei • Bactericidal: Bactericidal efficacy ≈ INH • Extra & intracellular bacteria Good sterilising property & resistance preventing action. • All sub populations; best on spurters MOA Rifampicin inhibits synthesis of R.N.A. It binds to β subunit of mycobacterial DNA dependent RNA polymerase & blocks its polymerising function Resistance develop due to mutation in rpo B gene( codes for RNA polymerase); X bind mammalian RNA polymerase (Basis for selectivity). Other bacteria Activity against other gram positive and gram negative bacteria • Staph • N. meningitidis • H. influenzae • E.coli • Kleibseilla • Psuedomonas • Proteus • Legionella • M. leprae is highly sensitive • MAC & other mycobacteria are moderately susceptible. Pk • Absorption Well absorbed from g.i tract Food also interferes with abs; empty stomach • Distribution widely distributed. Penetration intracellularly & enters • tubercular cavities • Caseous masses • Placenta • Metabolism Chiefly in liver to an active deacetylated metabolite. Which is excreted mainly in bile some in urine also. (30-70%). R and its metabolite undergoes enterohepatic circulation T1/2 varies from 2-5 hrs HEPATITIS a major adverse effects: effect Adverse Urine and secretions become orange-red in colour • Cutaneous syndrome • Flu syndrome • Abdominal syndrome SERIOUS BUT RARE • Respiratory syndrome: breathlessness • Purpura, haemolysis, shock and renal failure INTERACTIONS Rifampicin is a microsomal enzyme inducer • It induces several CYP 450 iso enzymes • Thus enhances its own metabolism as well as of other drugs including: Warfarin, OCPs, Corticosteroids, Anti-fungal drugs, Digitoxin, Protease inhibitors, NRTIs, etc. Increase dose; alternative method Why should Rifampicin not given with OCP? Other uses Leprosy Prophylaxis of meningococcal & H. influenzae meningitis & carrier state MRSA Brucellosis. PYRAZINAMIDE Weakly tuberculocidal More active in acidic medium More lethal to intracellular bacteria & bacteria at the site of inflammation Highly effective during 1st 2 months By killing the residual intracellular bacteria it has good sterilizing activity • Its inclusion has enabled duration of treatment to be shortened & reduced risk of relapse One third reduction in the duration of anti-TB therapy & a two third reduction in TB relapse • This led to reduction in duration of therapy to 6 mths, producing the short course ctx MOA Pyrazinamide Mycobacterial Pyrazinamidase Pyrazinoic Acid (gets accumulated in acidic medium) Inhibits Mycolic Acid Synthesis + • Pyrazinoic acid also disrupts mycobacterial cell membrane and its transport function • Resistance develops rapidly if used alone & is due to mutation of gene pncA • Absorption : Well absorbed orally • Distribution : good penetration to all body tissue & CSF • Metabolism: extensively in liver • Excreted in urine T1/2 6-10hrs Dose: 25-30mg/kg/dayAdverse effects: Hepatotoxicity (dose dependent); occurs at 40mg/kg/day; hepatic disease in 15%. Regimens employed currently 15-30 mg/kg/day are much safer. Hyperuricemia; inhibits excretion of urates. In nearly all patients. May ppt acute episodes of gout. Arthralgia, nausea, vomiting, dysuria, malaise and fever, loss of diabetes control ETHAMBUTOL[E] Only Tuberculostatic drug among 1st line drugs. Added to RHZ hastens the rate of sputum conversion and prevents the development of resistance. Primarily added for this reason. MOA E Inhibits Mycobact. Arabinosyl Transferase III Arabinogalactan synthesis Essential component of Myco. Cell wall; disrupts the assembly of mycobacterial cell wall. PK • • • • Absorption: Well absorbed from g.i.t. Distribution : Widely distributed T1/2 ~ 4hrs Excretion: Glomerular filtration & tubular secretion Dose to be reduced in Renal failure Side effects: Loss of visual acuity (reversible) loss of color vision Field Defect due to optic neuritis Dose & duration dependent toxicity. Pt should be instructed to stop the drug at first indication of visual impairment. Visual toxicity: reversible • Contra-indication; In children <6yrs and Creatinine clearance <50ml/min • Hyperuricaemia FQs • • • • Ofloxacin Levofloxacin Ciprofloxacin Moxifloxacin New potent oral mycobactericidal drugs • Primary indication Drug resistant TB key component of all regimens for MDR TB • Alternative to first line Substitution of Ethambutol with Mfx has been found to enhance rate of bacterial killing & cause faster sputum conversion Possibility of decreasing the duration of treatment to less than 6 months • Mfx is the most active FQ against M. TB • Lvx is more active than Ofx & Cfx Dose: • Ofloxacin: 800mg OD • Levofloxacin 750 mg OD • Moxifloxacin 400mg OD Goal of AT CTx • Kill dividing bacilli • Kill persisting bacilli • Prevent emergence of resistance. Goal of AT CTx Kill dividing bacilli • Reduce bacillary load • Achieve quick sputum negativity • Patient non-contagious • Transmission interupted • Quick symptomatic relief Kill persisting bacilli • Effect cure & prevent relapse Goal of AT CTx Prevent emergence of resistance. • So that bacteria remain susceptible to the drugs Short Course Chemotherapy Who has introduced 6-8 months multidrug “short course” regimens under DOTS programme. •4 drugs Multidrug therapy H R Z E 4 drugs/Multidrug therapy Why????? • Use of single drug in tuberculosis results in the emergence of resistant organisms and relapse in almost 3/4th of patients. • Combination: • H & R most potent bactericidal Combination synergistic Duration of therapy shortened from >12 months to 9 months • Z acts on intracellular bacteria It has very good sterilizing activity Addition of Pyrizinamide further reduces duration from 9 to 6 months • E is bacteriostatic mainly serves to prevent resistance and may hasten sputum conversion • Single daily dose • AKT-4 • • • • Cost Convenience Feasibility Decreased resistance TREATMENT CATEGORIES CATEGORY-I CATEGORY-II New cases Previously treated patient. Category I • New case • Sputum positive for Mycobacterium TB Category II • • • • • • Smear positive TB patients Exposed to ATT in the past Did not complete the course Or took irregular medication Or relapsed after responding Failed to respond; failures Higher risk of harbouring DR bacilli Intensive phase Two phases Continuation phase Intensive phase • 4-5 drugs • 2-3 months • Rapidly kill the bacilli Bring about sputum conversion Afford symptomatic relief Continuation phase • 2-3 drugs • 4-5 months Remaining (few) bacilli eliminated So that relapse does not occur Category Intensive phase Continuous phase Duration (months) Comment I 2 HRZE daily 4 HR daily 6 Optimal 2 HRZE daily 4 HR thrice weekly 6 Acceptable if DOT ensured 2 HRZE thrice weekly 4 HR thrice weekly 6 Acceptable if DOT ensured 2 HRZES daily + 1 HRZE daily 5 HRE daily 8 For patient with low/medium risk of MDR-TB. Emperical (Standardized) MDR regimen Emperical (Standardized) MDR regimen 18-24 Till DST II For patient with high risk of MDRTB. Failures, 2nd default, contact of MDR th Tt of TB Guidelines, 4 edition (2010), WHO , Geneva. Category two • Thrice weekly option not available for retreatment categories • Assess risk of MDR-TB (DST) Recommended doses Drug Daily dose 3 times per week dose mg/kg maximum mg/kg maximum Isoniazid 5(4-6) 300 mg 10(8-12) 900 mg Rifamin 10(8-12) 600 mg 10(8-12) 600mg Pyrazinamide 25(20-30) 35(30-40) Ethambutol 15(15-20) 30(25-35) Streptomycin 15(12-18) 15(12-18) 1000mg Tt of TB Guidelines, 4th edition (2010), WHO , Geneva. Multiple Drug Resistance(MDR) • Defined as Resistance to both Isoniazid and Rifampin (compulsorily) and number of other(1st line drugs) • Rapid course With worse outcomes • 2.8% of all new cases • 12-17% of retreatment cases • Treatment is difficult as second line drugs are less efficacious, less convenient, more expensive and toxic for longer duration General principles MDR Regimen • At least 4 drugs • Include drugs from group I to group IV Gp I drugs (except H;R) 2 + one injectable; Gp II 1 One FQ; Gp III 1 One/two Gp IV drugs 2 General principles Gp I drugs 2; (except H;R) Gp II one injectable 1 Gp III One FQ 1 One/two Gp IV drugs 2 Standardized RNTCP regimen for MDR-TB Intensive phase 6 drugs; 6-9 months; Continuous Phase 4 drugs; 18 months • • • • • • • • • • Kanamycin Ofloxacin Ethionamide Cycloserine Pyrazinamide Ethambutol Ofloxacin Ethionamide Cycloserine Ethambutol + Pyridoxine 100 mg/day RNTCP DOT plus guidelines;2010. Extensively Drug Resistant(XDR) • This term is applied to bacilli that are resistant to at least four most effective cidal drugs, i.e. Cases resistant to INH Rifampicin Flouroquinolone and one of Kanamycin/Amikacin/Capreomycin • Virtually untreatable • Mortality is very high, particularly among HIV positive patients. detected or diagnosed • Standardized MDR regimen must be stopped immediately • Expert panel may decide on instituting treatment with group v drugs • Uncertain efficacy • Expensive Chemoprophylaxis • INH: 300mg daily X 6 months Children : 10 mg /Kg • INH resistance H(5 mg/kg)+R(10 mg/kg; max: 600 mg)X 3 months • If INH cannot be used : R X 4 months Whom • • • • Contacts of open cases Children with Mx positive & TB pt in the family Neonate of TB mother Pt of leukemia – Diabetes – Silicosis – HIV positive – C. steroid therapy; who show +ve Montoux Pregnancy • S contraindicated; ototoxicity USA • Z not recommended INDIA • Avoid Z • 2HRE+7HR • Treatment should not be withheld or delayed because of pregnancy • All pregnant women being treated with INH should receive pyridoxine 10-25 mg/day Lactation • All ATDs compatible with breast feeding • Full course should be given to mother Infant: • BCG vaccination • 6 month INH prophylaxis after ruling out active TB. Thanks for your attention