* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Viral virulence genes
Ebola virus disease wikipedia , lookup
Bacteriophage wikipedia , lookup
Social history of viruses wikipedia , lookup
Human Endogenous Retrovirus-W wikipedia , lookup
Introduction to viruses wikipedia , lookup
Viral phylodynamics wikipedia , lookup
Plant virus wikipedia , lookup
Virus quantification wikipedia , lookup
History of virology wikipedia , lookup
Negative-sense single-stranded RNA virus wikipedia , lookup
Oncolytic virus wikipedia , lookup
Papillomaviridae wikipedia , lookup
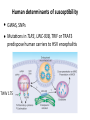
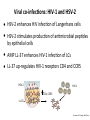
Viral virulence Lecture 15 Biology W3310/4310 Virology Spring 2014 Serious illness doesn’t bother me for long because I am too inhospitable a host. —ALBERT SCHWEITZER Animal models • • Human viruses in animals Animal viruses that resemble human infecNon Principles of Virology, ASM Press PVR PVR-‐Tg IFNα/β controls poliovirus tropism Viral virulence • • • Capacity of a virus to cause disease in a host Virulent vs avirulent or aQenuated virus Virulence can be quanNtated: -‐ Mean Nme to death -‐ Mean Nme to appearance of symptoms -‐ Measurement of fever, weight loss -‐ Measurement of pathological lesions (poliovirus); reducNon in blood CD4+ lymphocytes (HIV-‐1) Viral virulence Principles of Virology, ASM Press Viral virulence is a rela9ve property • Influenced by dose, route of infecNon, species, age, gender, and suscepNbility of host • • Cannot compare virulence of different viruses For similar viruses, assays must be the same Virulence depends on route of inocula9on Principles of Virology, ASM Press Viral virulence • Major goal of virology is to idenNfy viral and host genes that determine virulence • Virulence genes usually idenNfied by mutaNon: a virus that causes reduced or no disease in a specified system Iden9fying virulence genes Principles of Virology, ASM Press Viral virulence genes • • • • • • Viral replicaNon Invasiveness Tropism Modify the host defense mechanisms Enable the virus to spread in the host Intrinsic cell killing effects Virulence determinants may not encode proteins • AQenuated Sabin vaccine strains of poliovirus contain a mutaNon in the 5’-‐noncoding region that reduces neurovirulence • DeleNons within the long poly(C) tract in the 5’-‐ noncoding region of mengovirus reduce virulence in mice C C 500 A C AA •GC C• G •A U •G 490 CG•C C A A A (2) C•G G•C 510 G (1) G•C A•U C•G UC 480 G G U 472U (3) UA A CCAUGG A •• •••• • C• A 470 AGGUGCC C C• G A G U G C U A AA •A 530 ACGCG 520 U A C Principles of Virology, ASM Press Poliovirus replica9on in mouse brain Principles of Virology, ASM Press Gene products that modify host defense • Immune modulators -‐ -‐ -‐ -‐ • Apoptosis, autophagy Virokines and viroceptors Complement binding proteins Modifiers of MHC I, II pathways Not required for growth in cell culture Viral virulence genes Gammaherpesvirus 68 M3 gene encodes a chemokine receptor Principles of Virology, ASM Press Toxic viral proteins NSP4 nonstructural glycoprotein of rotaviruses: viral enterotoxin TRIM5α, a cellular virulence factor • • • Old world monkeys are not infected by HIV-‐1 Virions enter but encounter a block before RT RestricNon mediated by species-‐specific protein TRIM5α that acts on the viral capsid J. Cell Biol. Vol. 199 No. 3 407–412 • miR-‐122, liver-‐specific miRNA required for HCV replicaNon • AnN-‐miR-‐122 reduces HCV yield in chimpanzees Mechanisms of cell injury by viruses • CytolyNc viruses: cytopathic effects • Viral inhibiNon of host protein and RNA synthesis, leads to loss of membrane integrity, leakage of enzymes from lysosomes, cytoplasmic degradaNon • SyncyNum formaNon by enveloped viruses (parainfluenza, HIV) • Apoptosis, necrosis, pyroptosis Immunosuppression • Some virus infecNons suppress the immune response globally • Mechanisms -‐ -‐ ReplicaNon in one or more cells of immune system -‐ Viral proteins acNng as viroceptors or virokines (immune modulators) PerturbaNon of cytokine homeostasis and intracellular signaling Immunosuppression during measles infec9on Measles virus immunosuppression • DendriNc cells and monocytes are infected, anNgen-‐ presenNng acNvity compromised • • CirculaNng T lymphocytes are decreased by 50% Aberrant cytokine responses: IL-‐4 and IL-‐10 increased, skews Th1 to Th2 and reducing macrophage acNvaNon Examples of immunosuppression Virus Measles Rubella HIV Disease Measles Cells infected ManifestaNon Monocytes, DC Reduced DTH Thymic Enhanced infecNons epithelial cells Rubella Lymphoid cells AIDS CD4+ T cells monocytes Persistent rubella infecNon OpportunisNc infecNons Neoplasia Genes that control host suscep9bility to viral disease • flv gene of mice determines suscepNbility to flavivirus • flv encodes 2’-‐5’-‐oligo(A) synthetase • No known role in human infecNons Human determinants of suscep9bility • • • • Ccr5-‐delta32 mutaNon protects vs HIV-‐1 infecNon Present in 4-‐16% of European descent Stem cell therapy cured German AIDS paNent HIV gets the zinc finger: hQp://www.virology.ws/ 2014/03/19/hiv-‐gets-‐the-‐zinc-‐finger/ Principles of Virology, ASM Press Herpes simplex encephali9s • Rare and potenNally fatal CNS infecNon, ~1 case/ 250,000/yr • • 70% mortality if untreated Two peaks of incidence: 6 mo -‐ 3 yr (primary infecNon) and >50 yr (reacNvaNon from latency) Principles of Virology, ASM Press Human determinants of suscep9bility • • GWAS, SNPs MutaNons in TLR3, UNC-‐93B, TRIF or TRAF3 predispose human carriers to HSV encephaliNs TWiV 175 Influenza severity and IFITM3 • • IFITM3 is an ISG encoding a transmembrane protein • Humans hospitalized with severe influenza are enriched for a mutaNon in IFITM3 gene EssenNal for controlling influenza morbidity and mortality in mice IFITMs also inhibit dengue, HCV, Ebola Virus suscep9bility mapping to MHC Elite controllers • Long-‐term nonprogressors: low HIV loads without ARV • • MulNple traits responsible Associated with specific MHC I alleles (HLA-‐B57) Principles of Virology, ASM Press Viral co-‐infec9ons: HIV-‐1 and HSV-‐2 • • HSV-‐2 enhances HIV infecNon of Langerhans cells • • AMP LL-‐37 enhances HIV-‐1 infecNon of LCs HSV-‐2 sNmulates producNon of anNmicrobial pepNdes by epithelial cells LL-‐37 up-‐regulates HIV-‐1 receptors CD4 and CCR5 HSV-‐2 HIV-‐1 CD4, CCR5 LL-‐37 Principles of Virology, ASM Press Age • Very young and very old humans most suscepNble to disease • Young -‐ immaturity of immune response; greater freedom from immunopathology -‐ LCM i.c. adult mice lethal; infant mice survive (T cell response) • Old -‐ less elasNc alveoli, weaker respiratory muscles, diminished cough reflex Influenza, US, 1911-‐1915 Age excep9ons • Poliovirus, mumps virus, measles virus infecNons milder at young age; beQer balance of protecNve and pathogenic immune response at this age? • 1918 influenza pandemic lethal for very young, very old, and unexpectedly for young adults 18-‐30 yr old US 1911-‐1915 US 1918 Perhaps lacked protecNve immunity which would be conferred by previous infecNon with related virus Principles of Virology, ASM Press Other determinants • Males slightly more suscepNble to viral infecNons than females • Pregnancy: hepaNNs A, B, E, influenza more lethal, polio more common • MalnutriNon increases suscepNbility because physical barriers and immune response are compromised -‐ For this reason, measles is 300 Nmes more lethal in developing countries than Europe, N. America Other determinants • CigareQe smoking increases suscepNbility to respiratory infecNons • • Air polluNon increases respiratory disease Stress causes increased suscepNbility Chest 118:1150 (2000)