* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Chapter 2
Survey
Document related concepts
Transcript
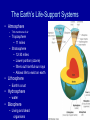
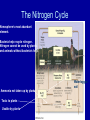
Science, Matter, Energy and Ecosystems Chapter 2 Pages 16-45 Matter and Energy • Read section 2-2 on Matter and Energy • Background to many ES issues and future chapters • We will discuss some but not all Science and Critical Thinking Constructing the Hypothesis • The goal of science is to discover facts about the natural world and the principles that explain these facts. • How does one “measure” the natural world? Use senses, see, hear, feel, taste smell, as well as tools to extend these senses – Observations • Can quantify, through statistics can validate – Scientific Knowledge is ultimately traced to Observations Constructing the Hypothesis The scientific method can be best described as procedures used to learn about our world. Science cannot prove or disprove nonquantifiable factors, such as ESP. Constructing the Hypothesis • Must be stated in a way that allows them to be tested. • A testable hypothesis is one that at least potentially can be proved false. Constructing the Hypothesis • For example: – There are no mermaids in the sea • This is testable and can be proven false by finding a mermaid – There are mermaids in the sea • This cannot be proven false, as the true believer would say “They are there, you just didn’t find them” Constructing the Hypothesis • Variables are factors that might affect observations • Models with variables one can alter – Laboratory • Ecological models – difficult to alter the variables. Often only observations to determine differences based on variability. • In science, no absolute truths. No hypothesis can be absolutely proved true. • Make best decisions with available evidence. •Scientific hypotheses – an unconfirmed explanation of an observation that can be tested •Scientific method – used to test hypotheses – ways scientists gather data, formulate and test hypotheses. •Peer review and publication – widely accepted – leads the scientific theories and laws. •Scientific theories – description of what we find happening through repeated observations – verified and credible hypothesis •Scientific (natural) laws – description of what we find happening, and is proven over and over •Frontier science – preliminary results – often subject to news stories •Junk Science – no peer review Levels of organization in nature. The shaded portion is the five levels that ecology is based upon. What is Matter? • Atoms, ions and molecules • Anything that has mass and takes up space. • Two forms: – Element – distinctive building blocks of matter that make up every material substance – Compound – two or more different elements held together by chemical bonds What is Matter? • Organic compounds – Compounds containing carbon atoms combined with each other and with atoms of one or more other elements such as hydrogen, oxygen, nitrogen, sulfur, phosphorus, chlorine, and fluorine. • Inorganic compounds – All compounds not classified as organic compounds. The Law of Conservation of Matter • Matter is not destroyed • It only changes form • There is no “away” – atoms are not destroyed, just rearranged. • What are some examples of matter changing form? First Law of Thermodynamics • Energy is neither created nor destroyed • Energy only changes form • You can’t get something for nothing – Or “There is no such thing as a free lunch!” • ENERGY IN = ENERGY OUT Energy • Kinetic – Wind – Electicity – Flowing water • Potential – Water behind a dam – Gasoline in your car – Unlit match Second Law of Thermodynamics • In every transformation, some energy is converted to heat • You cannot break even in terms of energy quality Waste energy is low quality and cannot be reused Second Law of Thermodynamics • What are some other examples of the Second Law of Thermodynamics? Water is heated due to energy loss from the flowing water and turbines 20-25% of the chemical energy in gasoline is converted to mechanical energy. The rest is lost into the environment as low quality heat energy. 5% of electricity is changed into useful light. 95% is lost as low-quality heat. • Photosynthesis is the process of converting solar energy into chemical energy stored in food • CO2 + H20 ---> C6H12O6 + O2 • Respiration is the process of releasing chemical energy stored in food to be used by living things. • C6H12O6 + O2 ---> CO2 + H20 Ecological Concepts • Ecology: Study of how organisms interact with each other and with their non-living surroundings. • Eco - is from the Greek word “Oikos” for house The Nature of Ecology Levels of study in Ecology: • Organisms – single animal • Populations – same species • Communities – pop’ns living together • Ecosystems – community + physical environment • Biosphere – all the earth’s ecosystems The Earth’s Life-Support Systems • Atmosphere – Thin membrane of air – Troposphere • 11 miles – Stratosphere • 12-30 miles • Lower portion (ozone) • filters out harmful sun rays • Allows life to exist on earth • Lithosphere – Earth’s crust • Hydrosphere – water • Biosphere – Living and dead organisms Natural Capital: Sustaining Life of Earth • One-way flow of energy from Sun • Cycling of crucial elements • Gravity Solar Capital: Flow of Energy to and from the Earth Greenhouse gasses water vapor CO2 Methane Ozone Increases kinetic energy, Helps warm troposphere. Allows life to exist (as we know it) on earth. As greenhouse gasses increase, temperature of troposphere increases. Ecosystem Components • Abiotic factors • Biotic factors • Range of tolerance for each species – what factors are important for… Ecosystem Components • Limiting factors determines distributions Law of Tolerance • The existence, abundance and distribution of a species is determined by levels of one or more physical or chemical factors. Common limiting factors • Limiting factors – more important in regulating population growth than other factors. • Terrestrial ecosystems (on land) – precipitation – temperature – soil nutrients • Aquatic ecosystems – – – – – temperature sunlight nutrients dissolved oxygen salinity Biological Components of Ecosystems • Producers (autotrophs) • Consumers (heterotrophs) – Herbivores, carnivores, omnivores – Decomposers and detritivores • detritus = dead organic material Biodiversity • Genetic diversity – variety of genetic material within a species or a population • Species diversity – the number of species present in different habitats • Ecological diversity – the variety of terrestrial and aquatic ecosystems found in an area or on earth • Functional diversity – biological and chemical processes needed for the survival of species, communities and ecosystems Energy Flow in Ecosystems • Food chains – sequence of organisms which is a source of food for the next. • Food webs – most species participate in several food chains (they don’t just eat one thing!). • Trophic levels – each step in the flow of energy through an ecosystem (feeding level) Food Chains and Energy Flow in Ecosystems Ecological Pyramids • Pyramid of energy flow • Ecological efficiency • Pyramid of biomass • Pyramid of numbers Food webs • reality tends to be more complex than a linear food chain Primary Productivity of Ecosystems • Gross primary productivity (GPP) • The rate at which an ecosystem's producers capture and store a given amount of chemical energy as biomass in a given length of time. • Net primary productivity (NPP) • Rate at which all the plants in an ecosystem produce net useful chemical energy; equal to the difference between the rate at which the plants in an ecosystem produce useful chemical energy (gross primary productivity) and the rate at which they use some of that energy through cellular respiration. • (NPP = GPP – Respiration) Net Primary Productivity comparison Soils • Importance • Provides most of the nutrients for plant life • Cleans water • Decompose and recycle biodegradable wastes • Maturity and Horizons • • • • Surface litter layer Top soil layer (humus) Sub soil Parent material • Variations with Climate and Biomes • Variations in Texture and Porosity Soil Profiles in Different Biomes Matter Cycling in Ecosystems Biogeochemical cycles – global cycles recycle nutrients through the air, land and water Cycles are driven directly or indirectly by solar energy and gravity • Hydrologic cycle (H2O) • Carbon cycle • Nitrogen cycle • Phosphorus cycle Hydrologic (Water) Cycle Human Influence on the Water Cycle • • • • • Water withdraw from lakes and streams Clear vegetation Construct impervious surfaces Fill wetlands Modify water quality by adding nutrients The Carbon Cycle (Marine) Based on Carbon Dioxide Terrestrial producers remove CO2 from the air; aquatic producers remove it from the water. Through photosynthesis, Converts to carbohydrates. O2 consuming producers respire,breaking carbohydrates back to CO2. CO2 not released until burned. The Carbon Cycle (Terrestrial) Human Influence on the Carbon Cycle • Clear trees and other plants, often times permanently • Burning fossil fuels and wood • Increased CO2 in the troposphere enhance natural greenhouse effect • Results in global warming The Nitrogen Cycle Atmosphere’s most abundant element. Bacteria help recycle nitrogen. Nitrogen cannot be used by plants and animals without bacteria’s help. Waterlogged soil Ammonia not taken up by plants Toxic to plants Usable by plants Human Influence on the Nitrogen Cycle • Add large amounts of nitric oxide by burning fuel • Gas converted to nitrogen dioxide gas and nitric acid (acid rain) • Add nitrous oxide through anaerobic bacteria breaking down livestock wastes (global warming). • Release nitrogen stored in soils and plants by destroying forests, grasslands and wetlands. • Add excess nitrates for agriculture • Remove nitrogen from topsoils through harvesting various crops The Phosphorus Cycle Slow Bacteria not a major player Washes from the land into streams, then the sea. Can be deposited as sediment and remain for millions of years. Often a limiting factor for plant growth on land. Also limits growth in lakes And streams because phosphate salts are only slightly soluble in water. Fig. 4-33 p. 82 Human Influence on the Phosphorus Cycle • We mine large quantities of phosphate rock to make inorganic fertilizers. • We reduce the available phosphate in tropical soils by clearing tropical forests. • We disrupt aquatic systems with phosphates from runoff of animal wastes and fertilizers, and sewage systems.