* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download unit 4: chemical reaction rates
History of electrochemistry wikipedia , lookup
Chemical industry wikipedia , lookup
Multi-state modeling of biomolecules wikipedia , lookup
Asymmetric induction wikipedia , lookup
Artificial photosynthesis wikipedia , lookup
Chemical potential wikipedia , lookup
Isotopic labeling wikipedia , lookup
Water splitting wikipedia , lookup
Marcus theory wikipedia , lookup
History of chemistry wikipedia , lookup
History of molecular theory wikipedia , lookup
Process chemistry wikipedia , lookup
Photoredox catalysis wikipedia , lookup
George S. Hammond wikipedia , lookup
Hydrogen-bond catalysis wikipedia , lookup
Nucleophilic acyl substitution wikipedia , lookup
Evolution of metal ions in biological systems wikipedia , lookup
Electrolysis of water wikipedia , lookup
Biochemistry wikipedia , lookup
Photosynthetic reaction centre wikipedia , lookup
Acid dissociation constant wikipedia , lookup
Rate equation wikipedia , lookup
Stability constants of complexes wikipedia , lookup
Physical organic chemistry wikipedia , lookup
Atomic theory wikipedia , lookup
Metalloprotein wikipedia , lookup
Strychnine total synthesis wikipedia , lookup
Determination of equilibrium constants wikipedia , lookup
Click chemistry wikipedia , lookup
Bioorthogonal chemistry wikipedia , lookup
Acid–base reaction wikipedia , lookup
Chemical reaction wikipedia , lookup
Electrochemistry wikipedia , lookup
Chemical thermodynamics wikipedia , lookup
Lewis acid catalysis wikipedia , lookup
Transition state theory wikipedia , lookup
Stoichiometry wikipedia , lookup
MANUAL
CHEMICAL REACTIONS
Apply knowledge of chemical reactions in a processing
environment
Unit Standard 244241
NQF Level 3
Credits: 6
Compiled by:
Kgomotso Matobole
Rev.1 – September 08
Moderated by:
Learner Name:
Learner Number:
Rev.1 – Sept 08
Sparrow Consulting © September 08
Table of Contents
UNIT 1: ELEMENTARY CHEMICAL CALCULATIONS .................................................... 5
1.1
Instructions ...................................................................................................... 5
1.2
Introduction ...................................................................................................... 7
1.2.1
Atomic number and mass number ................................................................... 7
1.2.2
Isotopes ........................................................................................................... 9
1.2.3
Atomic mass and relative atomic mass ............................................................ 9
1.2.4
Relative formula mass ................................................................................... 10
1.2.5
Mole .............................................................................................................. 11
1.2.6
Avogadro's number ........................................................................................ 12
1.2.7
Molar volumes of gases ................................................................................. 13
1.2.8
Chemical equation based calculations ........................................................... 14
UNIT 2: ACIDS AND BASES .......................................................................................... 16
2.1
Instructions .................................................................................................... 16
2.2
Introduction .................................................................................................... 18
2.2.1
Properties of acids and bases ........................................................................ 18
2.2.2
Definitions for acids and bases ...................................................................... 18
2.2.3
Strong and weak acids .................................................................................. 20
2.2.4
Water ionisation and pH ................................................................................ 22
2.2.5
Acid reactions ................................................................................................ 24
2.2.6
Acid-base titration calculations ...................................................................... 26
2.3
Experiment .................................................................................................... 29
UNIT 3: OXIDATION-REDUCTION (REDOX) REACTIONS............................................ 31
3.1
Instructions .................................................................................................... 31
3.2
Introduction .................................................................................................... 33
3.3
Oxidation number .......................................................................................... 33
3.3.1
Rules for assigning oxidation numbers .......................................................... 34
3.4
Balancing redox reactions.............................................................................. 35
3.4.1
Oxidation number method.............................................................................. 35
3.5
Standard electrode potential .......................................................................... 39
3.5.1
Galvanic cell .................................................................................................. 39
3.6
Standard potentials ........................................................................................ 41
3.7
Experiment .................................................................................................... 44
UNIT 4: CHEMICAL REACTION RATES ........................................................................ 46
4.1
Manual
Instructions .................................................................................................... 46
2
US 244241
Rev.1 – Sept 08
Sparrow Consulting © September 08
4.1.1
Chemical reaction rates ................................................................................. 48
4.1.2
Factors affecting the rate of reaction .............................................................. 49
4.2
Experiment .................................................................................................... 51
UNIT 5: CHEMICAL EQUILIBRIUM ................................................................................ 53
5.1
Instructions .................................................................................................... 53
5.1.1
Equilibrium ..................................................................................................... 55
5.1.2
Equilibrium Constant...................................................................................... 56
5.1.3
Factors affecting chemical equilibrium ........................................................... 59
5.2
Experiment .................................................................................................... 61
Figures and tables
Figure 1: Periodic table of elements with atomic and mass numbers ................................ 8
Figure 2: Carbon isotopes ................................................................................................. 9
Figure 3: Acids turn litmus paper red, and bases turn litmus paper blue .........................19
Figure 4: Titration setup ...................................................................................................26
Figure 5: example of regular batteries ..............................................................................39
Figure 6: Simple galvanic cell .........................................................................................40
Figure 7: Rate of reaction curve .......................................................................................48
Figure 8: Equilibrium of a trioxide producing reaction .......................................................56
Table 1: List of elements with their atomic mass and number .........................................10
Table 2: Acids and bases with their conjugates...............................................................20
Table 3: Common strong acids .......................................................................................21
Table 4: The ionisation constants of common acids ........................................................22
Table 5: Hydronium and hydroxide ions concentrations in various solutions ....................22
Table 6: pH values of common acids and bases .............................................................23
Table 7: Reduction potentials at 25ºC .............................................................................43
Manual
3
US 244241
Rev.1 – Sept 08
Sparrow Consulting © September 08
Module overview
Introduction
This module provides an introduction to elementary chemistry as it is applied in the
process industry. In Unit 1 the learner is prepared to perform a range of elementary
chemical calculations. This includes calculating relative atomic masses, relative formula
masses, masses for a mole of different substances and the mass and percentages of
substances. In Unit 2 the learner is introduced to the properties of acids and bases and
provided with a general understanding of reactions with acids and bases. In Unit 3 the
principles and working of oxidation-reduction reactions are discussed. Unit 4 concerns
chemical reaction rates and its effect on the industry. In Unit 5 learners are introduced to
the principles of chemical equilibrium, factors that affect chemical equilibrium and the
effect it has on industry.
Learning outcomes
By the end of this module, you should be able to:
Perform elementary chemical calculations.
Demonstrate an understanding of acids and bases.
Demonstrate an understanding of oxidation-reduction (redox) reactions and their
industrial applications.
Demonstrate an understanding of chemical reaction rates.
Demonstrate understanding of chemical equilibrium.
US specific outcomes
The following specific outcomes are covered in this module:
SO1: Perform elementary chemical calculations.
SO2: Demonstrate an understanding of acids and bases.
SO3: Demonstrate an understanding of oxidation-reductions (redox) reactions and
their industrial applications.
SO4: Demonstrate an understanding of chemical reaction rates.
SO5: Demonstrate understanding of chemical equilibrium.
Manual
4
US 244241
Rev.1 – Sept 08
Sparrow Consulting © September 08
UNIT 1: ELEMENTARY CHEMICAL CALCULATIONS
1.1
Instructions
Ref. No
SO1 AC1-7
Resources
Learning materials
CCFO2, 3, 6, 7
Learning Methodology
Read through Unit 1 of the learning materials.
Make notes of things you do not understand and/
or need more information on.
Workbook
Assessment
N/ a
N/ a
Ex. 1
Ass. 1
Act. 1
SO1 AC1-7
Classroom
Learners attend a lecture on the following:
CCFO 2-7
Facilitator
atomic number, mass number, atomic mass,
mole and Avogadro constant
relative atomic masses
relative formula masses
isotopes
calculation of the masses for a mole of
different substances
calculation of the volumes for a mole of
different gases
calculation of the mass and percentages of
substances.
Manual
5
Act. 2
US 244241
Rev.1 – Sept 08
Sparrow Consulting © September 08
Ref. No
Resources
Learning Methodology
SO1 AC5-7
Classroom
In pairs, discuss and calculate the:
CCFO1-7
Facilitator
masses for a mole of different substances
Calculators
volumes for a mole of different gases
mass and percentages of substances.
SO1 AC5-7
CCFO1-7
Learning materials and
workbook
PoE
Facilitator/ SME
Manual
Revise the work that you have done up to this
point. Make sure that you have completed the
CCFO checklist and obtained the required
evidence for your PoE. If there is anything that
you do not understand, ask your facilitator.
6
Act. 3
Workbook
Assessment
Ex. 2
Ass. 1
CCFOs
CCFOs
Act. 4
US 244241
Rev.1 – Sep 08
Sparrow Consulting © September 08
1.2
Introduction
Chemistry has its own language and it is therefore important to familiarise yourself with
the terminology before you perform elementary calculations.
1.2.1
Atomic number and mass number
The atomic number of an atom is the number of protons in the nucleus of an atom. If the
atom is neutral, there are an equal number of electrons and protons in an atom. It is
important to note that the number of electrons is closely related to the element's
properties. Every atom has a unique atomic number. For example, the only element with
an atomic number of 6 is carbon. The atomic number also indicates the position of the
element on the periodic table. The symbol Z is used to represent an atomic number.
The mass number is the sum of the protons and neutrons in the nucleus. These neutrons
are the neutral particles also contained in the nucleus and are indicated by the symbol N.
Mass number is indicated by the symbol A. Therefore mass number can be calculated as
follows: A=Z+N.
It is a norm to represent the nuclear composition of an element (E) as ZA E. For instance,
the symbol
8
16O
means that the oxygen atom has a mass number of 16 and atomic
number of 8.
Figure 1 below shows the elements with their atomic and mass numbers:
Manual
7
US 244241
Rev.1 – Sep 08
Sparrow Consulting © September 08
Figure 1: Periodic table of elements with atomic and mass numbers
Manual
8
US 244241
Rev.1 – Sep 08
Sparrow Consulting © September 08
1.2.2
Isotopes
Isotopes are atoms that have the same atomic number, but a different atomic mass. They
have the same number of protons and electrons, but a different number of neutrons. For
example, most carbon has six protons and six neutrons, which gives it an atomic mass of
12. Some carbon atoms have an atomic mass of 14, implying that it has six protons and
eight neutrons. This is illustrated in Figure 2, where the red dots indicate the protons and
the grey ones the neutrons. All isotopes of the same element have the same chemical
properties.
Figure 2: Carbon isotopes
1.2.3
Atomic mass and relative atomic mass
By definition the atomic mass of the carbon isotope 12C is exactly 12 atomic mass units
(amu). It follows that 1 atomic mass unit = 1/ 12 x mass of one 12C atom. Since one
atom has a mass of 1.9926 x 10-26 kg, it follows that 1 amu = 1.9926 x 10-26/ 12 =
1.6605 x 10-27 kg. This value is known as the atomic mass constant, u. Therefore atomic
mass is the total mass of protons, neutrons and electrons in a single atom (when the atom
is motionless).
The relative atomic mass of an element is the ratio of the absolute mass of an atom of that
element to that of the atomic mass unit. The atomic mass of the carbon isotope 12C is
exactly 12 atomic mass units. It is therefore a relative number that indicates how many
times heavier an atom is than a carbon atom. This is not a whole number because the
atoms in nature have isotopes of different masses. It is represented by the symbol Ar.
Note that relative atomic mass does not have units.
Manual
9
US 244241
Rev.1 – Sep 08
Sparrow Consulting © September 08
The table below lists a few elements with their atomic mass and number:
Name
Symbol
Atomic number
Relative atomic mass
Hydrogen
H
1
1.008
Helium
He
2
4.003
Lithium
Li
3
6.939
Beryllium
Be
4
9.012
Boron
B
5
10.811
Carbon
C
6
12.011
Nitrogen
N
7
14.007
Oxygen
O
8
15.999
Fluorine
F
9
18.998
Table 1: List of elements with their atomic mass and number
1.2.4
Relative formula mass
The smallest units of chemical compound are called formula units, regardless of whether
they are molecules or ions. The total mass of all the atoms in a molecule is called the
formula mass. Relative formula mass can be calculated by adding up all the relative
atomic masses of the elements that make up the compound. It is represented by the
symbol Mr and it is measured in g/ mol.
Example
The relative formula of marble (CaCO3) can be calculated as follows:
Mr. (CaCO3)
Manual
=
[40+12+(3x16)]
=
100g/ mol
10
US 244241
Rev.1 – Sep 08
Sparrow Consulting © September 08
1.2.5
Mole
Scientists discovered that by simply determining the mass of the substance, it was
possible to count particles or atoms. A mole (mol) is the amount of a pure substance that
contains the same amount of chemical units as there are atoms in exactly 12 grams of
carbon, namely 12.
In order to avoid confusion, it is necessary to specify the elementary units, which may be
a molecule, an atom, an electron, an ion, etc. For example, a mole of hydrogen can mean
either a mole hydrogen atoms (H) or hydrogen molecules (H2).
The symbol n is used to represent the amount of the substance. It is critical to note that
the amount of the substance is not the same thing as the mass of the substance, because
the amount (n) of a substance is proportional to the mass (m) of the substance. This
proportionality can also be written as an equation:
n= m/ Mr
Note the units of mole: g/ g.mol-1= g.mol/ g = mol
Example
1
How many moles of atoms are there in (a) 50g of aluminium and (b) 0.55g of
chlorine?
(a)
n= m/ Mr
= 50/ 27
= 1.85 mol
(b)
n= m/ Mr
= 0.550/ 35.5
= 0.22 mol
2
How many moles of hydrogen molecules are there in 10g of water?
In each water molecule there are two hydrogen atoms, therefore each mole of
water contains 2 moles of hydrogen.
Mr (H2O) =(1x2) + 16
= 18g/ mol
n(H2O) =10/ 18
Manual
11
US 244241
Rev.1 – Sep 08
Sparrow Consulting © September 08
= 0.56 mol
In this case 0.56 mole of water contains 2 x 0.56 mole of H atoms, which works out
to be 1.12 mol.
3
What is the relative atomic mass of an element of 0.1 mol and a mass of 1.6g? Also
name the element.
M=m/ n
= 1.6/ 0.1
=16g/ mol
From the periodic table it can be seen that oxygen has a relative atomic mass of
16g/ mol.
1.2.6
Avogadro's number
The mass of a carbon atom was determined to be 1.9926 x 10-23g. Therefore, the number
of carbon atoms in 12g 6 12C is
= 12g/ 1.9926 x 10-23g
= 6.02x1023
This number is known as the Avogadro's number or Avogadro's constant (named after the
Italian scientist, Amadeo Avogadro). This number equals 1 mol.
Avogadro's number is represented by NA or L and is measured in units of mol-1 because it
is the number of elementary particles in one mol of a substance.
Example
1
How many molecules are there in 50g of carbon dioxide?
Take as Ar (C) =12 and Ar (O) =16 from the periodic table
Mr (CO2)
= (12 +2x16)
= 44g/ mol
n (CO2)
= 50g/ 44g.mol-1
= 1.14 mol
This carbon dioxide contains 1.14 mol x6.02x1023 molecules/ mol
= 6.86 x1023 molecules
Manual
12
US 244241
Rev.1 – Sep 08
Sparrow Consulting © September 08
2
How many atoms are there in 0.25 mol of copper?
= 0.25 mol x 6.02x1023 atoms/ mol
Number of atoms
= 1.51x1023 atoms
3
Calculate the mass of 2 x1024 aluminium atoms
n (Al) = 2 x1024 atoms/ 6.02x1023 atoms/ mol
= 3.32 mol
Ar (Al) = 27g/ mol
m (Al) = 3.32 mol x 27g/ mol
=89.7g
1.2.7
Molar volumes of gases
The molar volume of a substance is the volume occupied by one mole of that substance.
It can easily be calculated from the known density of the substance, since:
Density = Mass/ volume
Gases are of particular interest since their density is greatly dependent on temperature
and pressure. Using the results obtained from experiments performed with various gases,
it was determined that 1 mol of a gas occupies 22.414 dm3 (cubic decimetre) at standard
temperature and pressure (STP). Standard temperature occurs at 0º C and standard
atmospheric pressure is101.3kPa. To put it in another way, one mole of any gas at STP
will occupy a volume of 22.4 dm3.
Example
1
How many litres will 0.350 moles of HCl occupy at STP?
Use the short method to solve for x:
22.414 dm3/1 mol =x/ 0.35 mol
x=7.84 dm3
This gas occupies 7.84 litres.
2
Manual
Calculate the volume occupied by 5g of hydrogen gas at STP.
13
US 244241
Rev.1 – Sep 08
Sparrow Consulting © September 08
Mr (H2)
= 1x2
= 2g /mol
n (H2)
= 5/ 2
= 2.5 mol
Short method
22.414 dm³/ 1 mol = x/ 2.5mol
x=56.04 dm³
1.2.8
Chemical equation based calculations
In the industry, chemical equations are of great importance, because it allows you to
determine whether a certain chemical manufacturing process will produce the desired
product economically.
Chemical equations express the net change in composition
associated with a chemical reaction by showing the number of moles of reactants and
products.
1.2.8.1
Mass calculations
Example
Sodium chlorate decomposes into sodium chloride and oxygen according the following
chemical equation:
2NaClO3 => 2NaCl + 3O2
Calculate the mass of oxygen when 50g of sodium chlorate decomposes completely into
sodium chloride.
1
Calculate the formula masses for the substances concerned.
Mr (NaClOз)
Mr (O2)
2
= 103.5g/ mol
= 32g/ mol
Multiply the balancing number by the formula mass.
2NaClOз => 2NaCl + 3O2
Manual
2x103.5
3x32
=207
=96
14
US 244241
Rev.1 – Sep 08
Sparrow Consulting © September 08
3
Use the mass unit in the problem statement and calculate the mass of oxygen by
means of a simple proportion:
207g of NaClOз produces 96g of oxygen
Therefore 50g of NaClOз yields
(50g/ 207g) x 96g
= 23.1g of O2
1.2.8.2
Percentage calculations
Example
1
Calculate the percentage of hydrogen in sulphuric acid.
The formula for sulphuric acid is H2SO4
2
Mr (H2SO4)
= 98g/ mol
Mr (hydrogen)
= 2g/ mol
Calculate the unknown moles of NaOH.
moles
=
concentration x volume
n (NaOH) =
CV
=
(50x10-3 L) x 0.1mol/ L
=
0.005 mol
Therefore the percentage of H
= (2/ 98) x100%
=2.04 %
Manual
15
US 244241
Rev.1 – Sept 08
Sparrow Consulting © September 08
UNIT 2: ACIDS AND BASES
2.1
Instructions
Ref. No
SO2 AC1-6
Resources
Learning materials
CCFO2-4, 6, 7
SO2 AC1-4
CCFO1-7
Samples of household
acids and bases
Litmus paper
Learning Methodology
Read through Unit 2 of the learning materials.
Make notes of things you do not understand and/
or need more information on.
As an introduction to Unit 2, collect samples of
household acids and bases, such as lemons,
vinegar, soap and other household products and
bring it to the first lecture.
SO2 AC1-4
Classroom/ Laboratory
Attend a lecture on:
CCFO1-7
Materials and
equipment for the
experiments
the properties of acids and bases
strong and weak acids
the ionisation of water and the concept of pH.
Computer and
projector with
animations of the
experiments and/ or
PowerPoint slides of
the experiments
Manual
Workbook
Assessment
N/ a
N/ a
Act. 5
Ass. 1
During the lecture, you will observe and/ or
participate in experiments/ animations of the
experiments to help you understand the concept
of acids and basis.
Ex. 3
Act. 6
16
US 244241
Rev.1 – Sept 08
Sparrow Consulting © September 08
Ref. No
Resources
Learning Methodology
SO2 AC5, 6
Classroom
Attend a lecture on how to:
CCFO1-7
Facilitator
balance and explain a range of acid
reactions by using accepted chemical
techniques and notation
perform standardisation calculations.
SO2 AC1-6
CCFO1-7
Learning materials and
workbook
PoE
Facilitator/ SME
Manual
Revise the work that you have done up to this
point. Make sure that you have completed the
CCFO checklist and obtained the required
evidence for your PoE. If there is anything that
you do not understand, ask your facilitator.
17
Act. 7
Workbook
Assessment
Ex. 4
Ass. 1
CCFOs
CCFOs
Act. 8
US 244241
Rev.1 – Sept 08
Sparrow Consulting © September 08
2.2
Introduction
It was not until a few hundred years ago that it was discovered that vinegar and other sour
tasting foods have this taste because they are acids. The word acid comes from the Latin
term ‘acere’, which means sour.
2.2.1
Properties of acids and bases
Characteristics of acids:
releases hydrogen ions [H+] into water solution
corrodes active metals
turns a blue litmus paper red
tastes sour.
Characteristics of bases:
generate hydroxide ion [OH-] into water solution
turns red litmus paper blue
has a soapy ('slippery') feel
has a bitter taste.
It would be too dangerous to taste a liquid in order to find out if it is a base or
an acid. That is why chemists use indicators. These indicators change
colour when added to acids or bases.
2.2.2
Definitions for acids and bases
Bases are also known as alkalis and are the chemical opposite of acids. There are many
definitions for acids and bases; in this section we will define them by three well-known
definitions.
Arrhenius's definition
Bronsted-Lowery's definition
Lewis's definition
2.2.2.1
Arrhenius’s definition
Arrhenius's equation for an acid and a base is as follows:
Acid + Base ↔ Water + Salt
Manual
18
US 244241
Rev.1 – Sept 08
Sparrow Consulting © September 08
The equation above is used to explain a reaction called neutralisation.
Figure 3: Acids turn litmus paper red and bases turn litmus paper blue.
It is important to note that litmus paper is the most commonly used and oldest
indicator of acids and bases.
2.2.2.2
Bronsted-Lowery's definition
An acid is a material that donates protons [H+] and a base is a material that accepts
protons. The following equation is normally used to illustrate this process:
acid + base ↔ acid + base
Example
HCl + H2O ↔ HзO+ + ClEach base has a conjugate acid and each acid has it own conjugate base, which only
differs with a proton. From the example HCl is an acid, its conjugate base is Cl - and H2O
is the base with HзO+ as its conjugate acid.
The table below gives a few examples of acids and bases with their conjugates.
Manual
19
US 244241
Rev.1 – Sept 08
Sparrow Consulting © September 08
Acids
Conjugate base
Base
Conjugate acid
HCl
Cl-
OH-
H2O
HNOз
NOз-
NHз
NH4+
H2SO4
HSO4-
COз²-
HCOз-
HSO4-
SO4²-
HCOз-
H2COз
Table 2: Acids and bases with their conjugates
2.2.2.3
Lewis's definition
An acid is a material that accepts an electron pair and a base donates an electron pair.
When using this definition, an acid can also be called an electrophile and a base a
nucleophile. This makes it possible for many more reactions to be considered as acidbase reactions even if they do not occur in solution.
2.2.3
Strong and weak acids
Since an acid is a proton donor when dissolved in water, a hydrogen ion (proton) is
transferred to a water molecule. This reaction produces a hydronium ion (H3O+ or H+) and
a negative ion. This is generally represented by the equations below:
HX + H2O ↔= HзO+ + X-
or
HX ↔ H+ + X-
At a certain time virtually 100% of the acid will have reacted to produce these products.
This acid gives the maximum concentration of [H+] and is considered to be a strong acid.
A strong acid is an acid that dissociates completely or nearly completely in an aqueous
solution. For a strong acid, the concentration of HзO+ will be equal to [X-], meaning that
after equilibrium has been reached the concentration of the acid HX will be close to zero.
Table 5 shows the common acids that are almost hundred percent ionised, for example
when hydrogen chloride dissolves in water.
Manual
20
US 244241
Rev.1 – Sept 08
Sparrow Consulting © September 08
Chemical formula and names of acids
HBr
Hydrobromic acid
HCl
Hydrochloric acid
HI
Hydroiodic acid
HNOз
Nitric acid
HClO4
Perchloric acid
Table 3: Common strong acids
A weak acid is one that does not completely ionise in water. The degree to which the acid
dissolves is also measured by the equilibrium constant Ka When the equilibrium constant
is relatively large then the acid is strong and when equilibrium constant is small then the
acid is weak. A very small equilibrium constant means a very weak acid. Consider the
dissociation of a weak acid, namely hypochlorous acid:
HOCl + H2O ↔= HзO+ + OClWhen formulating the expression for the equilibrium constant for the reaction:
Ka = [HзO+] x [OCl-]/ [HOCl] x [H2O]
The molar concentration of water is always assumed to be 1, therefore:
Ka = [HзO+] x [OCl-]/ [HOCl]
The numerical value of the ionisation constant gives the measure of the acid strength.
The larger the Ka value, the stronger the acid will be. The Ka for strong acids is in the
vicinity of infinity, implying that the equilibrium lies far to the right.
Do not confuse strong and weak acids or bases with concentrated and
diluted acids or bases.
An acid or base is not necessarily stronger when it is present in a high
concentration. A diluted acid or base does not make that acid or base
weaker.
Manual
21
US 244241
Rev.1 – Sept 08
Sparrow Consulting © September 08
Acid
Formula
Ionisation constant Ka
Hydrochloric
HCL
107
Sulfurous
H2SO3
2.4 ×10-6
Phosphoric
H3PO4
7.5×10-3
Ammonia
NH3
1.8 x10-5
Hydrogen Sulfate ion
HSO4-
1.1×10-2
Hypochlorous
HCLO
3.5 x 10-8
Table 4: The ionisation constants of common acids
2.2.4
Water ionisation and pH
Water can act as a base or an acid and as such is termed amphitropic. Amphitropic
elements can either accept or donate a proton. The equation below, called the auto
ionisation of water, has the equilibrium constant Kw:
H2O + H2O ↔HзO+ + OHKw = [HзO+] x [OH-]
The dissociation constant (Kw), also known as the ion product, has the very small value of
10-14 at 25 ºC and gives us the concentrations of hydronium and hydroxide ions in pure
water, acidic and basic solutions. The table below indicates the range of hydronium and
hydroxide ion concentrations in various solutions.
Solution
Hydronium ion molar
concentration [HзO+]
Hydroxide ion molar
concentration [H-]
Neutral
1x10-7
1x10-7
Acidic
> 1x10-7
< 1x10-7
Basic
< 1x10-7
> 1x10-7
Table 5: Hydronium and hydroxide ions concentrations in various solutions
Another handy way of writing concentrations in terms of pH, which corresponds to the
concentration of hydronium ions in a solution, is:
pH = - log [H+]
Manual
22
US 244241
Rev.1 – Sept 08
Sparrow Consulting © September 08
The pH scale measures the acidity or alkalinity of a solution. Taking the exponent of the
HзO+ concentration and removing the negative sign will determine the pH of the
concentration. This gives you the pH of a solution. The pH scale ranges from 0 to 14,
where 7 is considered neutral ([HзO+] = [OH-]), less than 7 is acidic while more than 7
basic. The further away from 7 you are on the pH scale, the more acidic or basic the
solution. Therefore, a low pH means that the substance is more acidic, while a high pH
means that the substance is basic.
The table below contains examples of our everyday acids and bases and their pH values.
[HзO+]
pH
[OH-]
Example
1 X 100
0
1 X 10-14
Battery acid
1 X 10-1
1
1 X 10-13
Stomach acid
1 X 10-2
2
1 X 10-12
Lemon juice
1 X 10-3
3
1 X 10-11
Grapefruit
1 X 10-4
4
1 X 10-10
Tomato juice
1 X 10-5
5
1 X 10-9
Black coffee
1 X 10-6
6
1 X 10-8
Saliva
1 X 10-7
7
1 X 10-7
Pure water
1 X 10-8
8
1 X 10-6
Sea water
1 X 10-9
9
1 X 10-5
Baking Soda
1 X 10-10
10
1 X 10-4
Milk of magnesia
1 X 10-11
11
1 X 10-3
Ammonia solution
1 X 10-12
12
1 X 10-2
Soapy water
1 X 10-13
13
1 X 10-1
Bleaches
1 X 10-14
14
1 X 100
Caustic soda
Table 6: pH values of common acids and bases
Manual
23
US 244241
Rev.1 – Sept 08
Sparrow Consulting © September 08
From the theory above it can be concluded that the lower the pH value, the more acidic
the medium is and therefore the higher the value of the Ka will be.
2.2.5
Acid reactions
Acids reacts with many compounds, too many to look at in one experiment. In this section
we will discuss only five basic reactions. These reactions are discussed below.
2.2.5.1
Acid and metal reactions
Reactions with acids would normally involve a proton transfer (H+), but when an acid and
a metal react an electron transfer takes place. The hydrogen from the acid is displaced by
the metal, releasing hydrogen gas and producing a salt:
Acid + Metal ↔ A salt + Hydrogen
Example
H2SO4 + Mg ↔ MgSO4 + H2
2HCl + Mg ↔MgCl2 + H2Hydrogen causes bubbling during the reaction. It can be detected by holding a burning
match close to the mouth of the gas collection tube, which will cause an explosive sound.
2.2.5.2
Acid and carbonate reactions
When an acid reacts with a carbonate it produces water, salt and carbon dioxide.
Acid + Carbonate ↔ A salt + Water + Carbon dioxide
Example
2HCl + Na2CO3 ==> 2NaCl + H2O + CO2
2HNO3 + CaCO3 ==> Ca(NO3)2 + H2O + CO2
Carbon dioxide also causes bubbling. It can be detected using lime water, which will turn
clear lime water milky.
Manual
24
US 244241
Rev.1 – Sept 08
Sparrow Consulting © September 08
2.2.5.3
Acid and metal oxides reactions
Metal oxides are also bases. When they react with acids they form a salt and water,
generally:
Acid + Metal oxide ↔ A salt + Water
Example
H2SO4 + CuO ↔CuSO4+ H2O
2HNO3 + CuO ↔Cu(NO3)2+ H2O
2.2.5.4
Acid and metal hydroxide reactions
Metal hydroxide is also a special type of a base, because when it reacts with a base it
also produces a salt and water.
Acid + Metal hydroxide ↔ A salt + Water
Example
H2SO4 + 2NaOH ↔Na2SO4 + 2H2O
NaOH + 3HNO3 ↔NaNO3 + 2H2O
2.2.5.5
Acid and alkali reactions
Not all the bases are water soluble; those that are soluble in water are called alkalis.
When an acid reacts with an alkali it produces a salt and water.
Acid + Alkali ↔ A salt + Water
Example
HCl + NaOH ↔ NaCl + H2O
H2SO4 + 2KOH ↔K2SO4 + H2O
This is also called a neutralisation process because an alkali is a special type of a base.
Manual
25
US 244241
Rev.1 – Sept 08
Sparrow Consulting © September 08
2.2.6
Acid-base titration calculations
Titration is a method that is commonly used to determine the concentration of an unknown
liquid by comparing it with a known liquid. Acid-base titration involves the progressive
addition of a base of known concentration from the burette to the unknown volume of the
acid in the conical flask. This is depicted in the next figure. The volume of the acid and
base is precisely measured.
This process continues until the equivalence point is
reached. Equivalence point, which is shown by an indicator, is the point at which the
moles of H+ are equal to the moles of OH-.
Figure 4: Titration setup
This is the basis of all titration calculations. The following example illustrates how titration
calculations are performed.
Example
It was found that 50 mL of 0.10M NaOH neutralised 25.0mL of hydrochloric acid.
Determine the concentration of the acid used.
1
Write the balanced chemical equation for the reaction.
NaOH (aq) + HCl (aq) ↔ NaCl (aq) + H2O(l)
2
Calculate the unknown moles of NaOH.
moles
=
concentration x volume
n (NaOH) =
Manual
CV
26
US 244241
Rev.1 – Sept 08
Sparrow Consulting © September 08
3
=
(50x10-3 L) x 0.1mol/L
=
0.005 mol
From the balanced equation, find the mole ratio.
It is clear that the reactants are consumed on a 1:1 mole ratio.
4
Find the moles of the acid.
NaOH : HCl = 1:1
At equivalence point n(NaOH) = n(HCl) = 0.005 mol
5
Calculate the concentration of HCl.
n= 0.005 mol, V = 25.0 x 10-3L
C= n/ VC
C(HCl) = 0.005 / 25.0 x 10-3
= 0.2 mol/ L
Example
In an experiment 100mL of 0.2mol-1 L NaOH neutralised 50mL of sulphuric acid.
Determine the concentration of the acid.
1
Write the balanced chemical equation for the reaction.
2NaOH(aq) + H2SO4(aq) ↔ Na2SO4(aq) + 2H2O(l)
2
Calculate the unknown moles of NaOH.
moles =
concentration x volume
n(NaOH)
3
=
CV
=
(100x10-3 L) x (0.2mol/ L)
=
0.02 mol
From the balanced equation, find the mole ratio.
The reactants are consumed on a 2:1 mole ratio.
Manual
27
US 244241
Rev.1 – Sept 08
Sparrow Consulting © September 08
4
Find the moles of the acid.
NaOH : H2SO4= 2:1
At equivalence point n(H2SO4) = (0.5) n(NaOH) = 0.01 mol.
5
Calculate the concentration of H2SO4.
V = 50. x 10-3L
n = 0.01 mol,
C = n/ V
C(H2SO4) = 0.01/ 50 x 10-3
= 0.2 mol/ L
Example
In an experiment, it was found that 10mL of 0.025 mol-1 Ba (OH)2 neutralised 35 mL of
nitric acid. Determine the concentration of the acid.
1
Write the balanced chemical equation for the reaction.
Ba(OH)2(aq) + 2HNO3(aq) ↔ Ba(NO3)2(aq) + 2H2O(l)
2
Calculate the unknown moles of Ba (OH)2.
moles
=
concentration x volume
n(Ba(OH)2 =
3
CV
=
(10x10-3 L) x 0.025 mol/ L
=
2.5 x 10-4 mol
Calculate the moles of the acid.
Ba(OH)2:HNO3 = 1:2
At equivalence point n(HNO3) = 2 x n(Ba(OH)2 =5 x 10-4mol
4
Calculate the concentration of HNO3.
n =5 x 10-4mol; V = 35 x 10-3L
C = n/ V
C(HNO3) =5 x 10-4/ 35 x 10-3
Manual
28
US 244241
Rev.1 – Sept 08
Sparrow Consulting © September 08
= 0.0143 mol/ L
2.3
Experiment
The following experiment will be conducted in the chemistry laboratory so that you can
see how the concentration of an unknown acid solution can be determined.
Observe a demonstration of and/ or conduct the following
experiment.
Purpose
To determine the concentration of an unknown acid solution.
Apparatus and
materials
pipette bulb
burette clamp
50 ml graduated cylinder
100 ml graduated cylinder
125 ml Erlenmeyer flask
20 ml pipette
retort stand
spatula
wash bottle
unknown acid solution
0.1 M NaOH solution.
Correct PPE, including eye protection should be worn at all
Safety hazards
and precautions
times throughout the experiment.
If you spill the base or the acid solution on your skin, rinse with
water immediately.
Procedure
Rinse the 125 ml flask and 20 ml pipette with distilled water.
Rinse the pipette using a small volume of the unknown acid
solution.
Manual
Pipette 20ml of the unknown acid solution into the 125 ml flask.
29
US 244241
Rev.1 – Sept 08
Sparrow Consulting © September 08
Add about 20 ml of distilled water and a few drops of
phenolphthalein.
Record your observation of the colour of the solution.
Clean the burette with distilled water and rinse it with a small
volume of the NaOH solution.
Fasten the burette to the retort stand.
Pour the NaOH solution in a burette, making sure that the stop
cock is closed.
Place the 125 ml flask below it as shown in the sketch.
Perform the titration by slowly adding small amounts (1 ml) of
the NaOH solution into the unknown acid solution by opening
the burette to release the solution. Be careful not to add too
much at one time.
Record your observation of the colour of the solution as you add
more base.
When your solution has reached the equivalence point, the
solution should have a pale pink colour.
Record the amount of base you added to the acid and then
perform the calculations in the workbook to determine the acid
concentration.
Perform the experiment again to make sure you get same
results.
Sketch
Manual
30
US 244241
Rev.1 – Sept 08
Sparrow Consulting © September 08
UNIT 3: OXIDATION-REDUCTION (REDOX) REACTIONS
3.1
Instructions
Ref. No
SO3 AC1-5
Resources
Learning materials
CCFO2-4, 6, 7
Learning Methodology
Read through Unit 3 of the learning materials.
You can either work individually or in pairs. Make
notes of things you do not understand and/ or
need more information on.
Workbook
Assessment
N/ a
N/ a
Ex. 5
Ass. 1
Act. 9
SO3 AC1-3
Classroom
Attend a lecture on:
CCFO1-7
Facilitator
the principles of reduction and oxidation
reactions
the principles of standard electrode
potentials.
Experiment -Clean
plate of zinc, beakers,
zinc sulphate solution,
copper plate, copper
sulphate salt bridge,
cotton wool, voltmeter.
Computer and
projector with
animations of the
experiments and/ or
PowerPoint slides of
the experiments
Manual
During the lecture, you will observe and/ or
participate in experiments/ animations of the
experiments to help you understand the concept
of reduction-oxidation reactions.
31
Act. 10
US 244241
Rev.1 – Sept 08
Sparrow Consulting © September 08
Ref. No
Resources
Learning Methodology
SO3 AC4, 5
Classroom
Attend a lecture on how to:
CCFO1-7
Facilitator
balance Redox reactions by using the
oxidation states of the elements.
make predictions based on information
obtained from a redox table.
SO3 AC1-5
CCFO1-7
Learning materials and
workbook
PoE
Facilitator/ SME
Manual
Revise the work that you have done up to this
point. Make sure that you have completed the
CCFO checklist and obtained the required
evidence for your PoE. If there is anything that
you do not understand, ask your facilitator.
32
Act. 11
Workbook
Assessment
Ex. 6
Ass. 1
CCFOs
CCFOs
Act. 12
US 244241
Rev.1 – Sept 08
Sparrow Consulting © September 08
3.2
Introduction
Reduction-oxidation (redox) reactions are a group of reactions that are concerned with the
transfer of electrons between compounds.
These reactions always go together.
A
reduction reaction will not happen without an oxidation reaction happening simultaneously
in chemical equivalent quantities. When oxidation occurs, electrons are lost and electrons
are gained when reduction occurs. Hint: (LEO-loss of electrons is oxidation, GER- gain of
electrons is reduction). Each reaction on its own is called a half reaction.
A substance that is oxidised loses electrons and is called a reducing agent while the one
which is reduced gains electrons and is called an oxidising agent. For example, the
reaction between magnesium and oxygen atoms may be broken down into two half
reactions:
This process represents an oxidation half reaction with magnesium as a reducing agent.
This reaction represents a reduction half reaction with oxygen as an oxidising agent.
Figure 4: Corrosion of metals is a redox reaction that occurs naturally.
3.3
Oxidation number
The change in the oxidation number of a material provides a proper definition of oxidation
and reduction. The oxidation number is the charge an atom has if bonding electrons are
transferred completely to the atom with greater affinity for them in a particular situation.
Manual
33
US 244241
Rev.1 – Sept 08
Sparrow Consulting © September 08
The ratio by which the atoms will combine when forming compounds can be predicted by
these oxidation numbers. It means that the oxidation number is the charge an atom has if
it is in a compound composed of ions. In the case of oxidation, the oxidation number will
increase. The oxidation number will decrease when reduction occurs.
3.3.1
Rules for assigning oxidation numbers
The oxidation number of elements in their natural form is zero. For example, the oxidation
number of hydrogen in H2 or oxygen in O2 is 0.
An element in a monatomic ion has an oxidation number equal to the charge of the ion.
For example, aluminium and oxygen in Al2O3 have oxidation numbers of +3 and -2
respectively. The ions in this case are Al3+ and O2-.
Some elements have the same oxidation number in almost all their compounds. The 1A
metals always have an oxidation number of +1 while 2A elements always have an
oxidation number of +2 in their compounds. The most electronegative element, fluorine
always has an oxidation number of -1 in all its compounds. Oxygen is assigned -2 as its
oxidation number. Hydrogen, in its compound with metals is assigned -1 while with nonmetals it is assigned +1.
The sum of the oxidation numbers of atoms in a neutral species equals zero in an ion that
equals the charge on that species. We can use this principle to find oxidation number of
elements in polyatomic substance.
Application of this principle is illustrated by the
following examples.
Example
1
What is the oxidation number of manganese in MnO4-?
For MnO4-, oxygen’s oxidation number is taken as -2 and it should be realized that
the sum is -1
Therefore,
4 x (-2) +ox no (Mn) = -1
ox no (Mn) = +7
2
What is the oxidation number of sulphur in sulphuric acid?
In H2SO4, oxygen’s oxidation number is taken as -2, hydrogen as +1 and the sum
is 0.
Therefore,
Manual
4 (-2) +2(+1) + ox no (S) = 0
34
US 244241
Rev.1 – Sept 08
Sparrow Consulting © September 08
oxidation no (S) = +6
3.4
Balancing redox reactions
There are many methods used to balance redox reactions, however in this section we will
describe the use of the oxidation number method to balance these reactions.
3.4.1
Oxidation number method
The oxidation number method is illustrated by the examples below.
Example
Mn2+ is oxidised by NaBiO3 in acidic conditions according to this equation:
Mn2+ + BiO3- + H+ → MnO4- + Bi3+ + H2O
1
2
Determine the oxidation number of all elements on each side of the equation.
Ox no of reactants
Ox no of products
Mn
+2
+7
oxidised
Bi
+5
+3
reduced
O
-2
-2
-
H
+1
+1
-
From this, one can deduce that manganese was oxidised by freeing five electrons
and bismuth was reduced by accepting two electrons. To make an increase in
oxidation number equal a decrease in oxidation number, there must be five Bi
atoms reduced for two Mn atoms oxidised:
2Mn2+ + 5BiO3- + H+ → MnO4- + Bi3+ + H2O
3
From here the rest can be balanced by inspection and it is easy to do, giving:
2Mn2+ + 5BiO3- + 14H+ →2MnO4- + 5Bi3+ + 7H2O
Manual
35
US 244241
Rev.1 – Sept 08
Sparrow Consulting © September 08
Example
In an acidic medium, potassium permanganate oxidises chlorine ions to chlorine gas by
this chemical equation:
MnO4- + Cl- + H+→Mn2+ + Cl2 + H2O
1
What is the oxidation number of manganese in MnO4-?
For MnO4-, oxygen’s oxidation number is taken as -2 and it should be realized that
the sum is -1.
Therefore,
4 x (-2) +ox no (Mn) = -1
ox no (Mn) = +7
Example
In an acidic medium, potassium permanganate oxidises chlorine ions to chlorine gas by
this chemical equation:
MnO4- + Cl- + H+ → Mn2+ + Cl2 + H2O
1
2
Ox no of reactants
Ox no of products
Mn
+7
+2
reduced
Cl
-1
0
oxidised
O
-2
-2
-
H
+1
+1
-
In this case, the oxidation number of manganese decreases by five units while
chlorine's oxidation number increases by one unit.
To make an increase in oxidation number equal a decrease in oxidation number,
there must be five Cl atoms oxidised per Mn reduced. Thus:
MnO4- + 5Cl- + H+ → Mn2+ + Cl2 + H2O
3
Balance the rest by inspection, yielding:
MnO4- + 5Cl- + H+ → Mn2+ + 5/2 Cl2 + H2O
4
Manual
Or multiply through by a factor of two to eliminate fractional coefficients:
36
US 244241
Rev.1 – Sept 08
Sparrow Consulting © September 08
2MnO4- + 10Cl- + 16H+ → 2Mn2+ + 5Cl2 + 8H2O
Example
Consider this equation and balance it using the oxidation number method.
HNO3(aq)+H3AsO3(aq)→NO(g)+H3AsO4(aq)+H2O(l)
1
From the equation it looks like it will be difficult to do a balance on oxygen and
hydrogen. Let’s rather do a balance on nitrogen and arsenic atoms.
3
Ox no of reactants
Ox no of products
N
+5
+2
oxidised
As
+3
+5
reduced
From this, one can deduce that nitrogen was oxidised by losing 3 electrons and
arsenic was reduced by gaining two electrons in the process. To make an
increase in oxidation number equal a decrease in oxidation number, there must be
three As atoms reduced for every two N atoms oxidised:
2HNO3(aq)+3H3AsO3(aq)→ NO(g)+H3AsO4(aq)+H2O(l)
4
From here one can balance this equation by inspection, yielding:
2HNO3(aq)+3H3AsO3(aq)→NO(g)+3H3AsO4(aq)+H2O(l)
5
Balancing redox reactions using half equations.
An easy way of balancing redox reactions occurring in an aqueous solution
involves breaking up the equation into two half equations (reduction half reaction
and oxidation half reaction). The two reactions are balanced separately and then
combined to get the overall equation. There must be no net change in the number
of electrons in the overall equation.
In each half reaction:
Balance all other atoms except O and H.
O is balanced by adding H2O.
H is balanced by adding H+.
charges are balanced using electrons.
Manual
37
US 244241
Rev.1 – Sept 08
Sparrow Consulting © September 08
Example
Consider a reaction that occurs between chlorine and potassium permanganate:
MnO4-(aq) + Cl- (aq) →Mn2+ (aq)+ Cl2 (g)
1
Separate the equation into its half equations and work on them separately. Half
equation for reduction:
MnO4-(aq) → Mn2+ (aq).
2
From the equation we have the same number of manganese atoms on both sides,
but we have four oxygen atoms in excess. To balance the oxygen, we add an
appropriate number of H2O molecules; in this case we add four H2O molecules to
the right of the equation:
MnO4-(aq) → Mn2+ (aq) + 4H2O.
3
We add eight H+ ions to the left of the equation to balance hydrogen atoms that are
in excess on the right side of the half equation:
MnO4-(aq) + 8H+ (aq) → Mn2+ (aq)+ 4H2O.
4
Finally the charge must be balanced; we have +2 on the right and +7 on the left.
This +7 on the left comes from (+1x8) -1. Thus we add five electrons on the left to
balance the charge:
MnO4-(aq) + 8H+ (aq) + 5e- → + Mn2+ (aq) + 4H2O
5
equation 3.1
Next, we balance the oxidation reaction:
Cl- (aq) → Cl2 (g).
6
Lastly, we balance the charge; we have -2 on the left. Thus we add -2 on the right:
2Cl- (aq) → Cl2 (g) + 2e-
7
equation 3.2
We have two chlorine atoms on the right, thus we balance the atoms on the left by
multiplying the left side of the equation by two:
2Cl- (aq) → Cl2 (g).
8
Now, combine the two balanced half equations in such a way that the electron gain
equals the electron loss. So, we multiply equation 3.1 by 2 and equation 3.2 by 5.
10Cl- (aq) →2Cl2 (g) + 10e2MnO4- (aq) + 16H+ (aq) + 10e-→ 2Mn2+ (aq)+ 8H2O
10Cl- (aq) + 2MnO4- (aq) + 16H+ (aq)→2Cl2 (g) +2Mn2+ (aq) + 8H2O
Manual
38
equation 3.3
US 244241
Rev.1 – Sept 08
Sparrow Consulting © September 08
9
Equation 3.3 is our final equation.
From the above example, one would wonder why H2O and H+ molecules are used to
balance oxygen and nitrogen.
It is done because they are the only substances that
contain O and H in large quantities in aqueous solutions.
3.5
Standard electrode potential
Figure 5: example of regular batteries
A normal battery can be used to explain half reactions. The battery is also called an
electrochemical cell. In this type of cell, the production of electricity brings about a change
in chemical reaction. There are two types of electrochemical cells, namely a Galvanic
cell, where the reaction occurs spontaneously and an electrolytic cell where a nonspontaneous reaction occurs.
These cells contain electrodes where oxidation and reduction occur. Oxidation occurs at
the electrode called the anode and reduction occurs at the cathode. The cathode is a
positive electrode while an anode is a negative electrode.
A Galvanic cell is also called the voltaic cell and is commonly used because it is
spontaneous. In this section we will discuss this type of cell in its simplest form.
3.5.1
Galvanic cell
The following experiments will illustrate how a simple galvanic cell works.
Experiment
Conduct the following experiment.
Purpose
To illustrate how a simple galvanic cell works
Apparatus and
materials
clean plate of zinc
2 Beakers
zinc sulphate solution
copper plate
Manual
39
US 244241
Rev.1 – Sept 08
Sparrow Consulting © September 08
Procedure
copper sulphate solution
cotton wool
salt bridge.
Place a clean plate of zinc in a beaker containing zinc sulphate
solution and a copper plate in another beaker containing copper
sulphate.
Connect the two solutions with a salt bridge filled with a saturated
solution of KCl or KNO3 and plug it with cotton wool to prevent the
solution from running out. The functions of the salt bridge are:
to complete the circuit
to allow the movement of ions to ensure electrical neutrality and
to prevent direct contact between the two electrode solutions
(electrolytes).
The copper plate is connected to the positive terminal of the
voltmeter while the zinc is connected to the negative terminal. The
electrical potential difference between the redox pairs is measured
by the voltmeter.
Observation
The zinc electrode loses electrons and its mass decreases.
Electrons flow from the zinc electrode through the voltmeter.
A layer of copper deposits form on the copper electrode.
Conclusions
The zinc electrode is an anode because it loses electrons. Thus
oxidation occurs at this electrode by this half reaction:
Zn → Zn2+ + 2eThe electron is accepted by the Cu2+ ions in the solution:
Cu2+ + 2e-→Cu
Therefore, the copper electrode is a cathode because reduction
occurs in this electrode. This is depicted in Figure 6.
Figure 6: Simple galvanic cell
Manual
40
US 244241
Rev.1 – Sept 08
Sparrow Consulting © September 08
3.6
Standard potentials
The most important property of a voltaic cell to chemists is the voltage or the electrical
potential. This potential measures the driving force behind the reaction taking place in the
cell. The electrical potential is measured in volt (V). The potential of a single half cell
cannot be measured directly by a voltage measuring device; however, when two half cells
are connected to form a cell, the potential difference can be measured. In other words,
the potential difference between the anode and the cathode is measured.
A reduction half cell has a higher reduction potential than the half cell where oxidation
occurs, because it has a higher tendency to acquire electrons. The reduction potential of
a half cell measures the tendency of a half cell to occur as reduction. Cell potential is the
difference between the reduction potential of the two half cells at hand:
E ocell = E ocathode- E oanode
E ocell, the standard cell potential, is the measure of the cell potential under standard
conditions, which are:
for gases, a pressure of 1 atmosphere
a temperature of 25 oC
an ion concentration in the half cell is 1M.
As mentioned previously, the half cell potentials cannot be measured, so scientists have
chosen a reference electrode. This electrode is the standard hydrogen electrode and was
assigned a value of 0.00V. All other potential measurements can be made against this
electrode potential.
The table below gives the standard reduction potentials of different chemicals in water
solution at 25 oC.
Manual
Half-Reaction
E 0(V)
Li+(aq) + e- → Li(s)
-3.05
K+(aq) + e- → K(s)
-2.93
Ba2+(aq) + 2e- → Ba(s)
-2.90
Sr2+(aq) + 2e- → Sr(s)
-2.89
Ca2+(aq) + 2e- → Ca(s)
-2.87
Na+(aq) + e- → Na(s)
-2.71
41
US 244241
Rev.1 – Sept 08
Sparrow Consulting © September 08
Mg2+(aq) + 2e- →Mg(s)
-2.37
Be2+(aq) + 2e- → Be(s)
-1.85
Al3+(aq) + 3e- → Al(s)
-1.66
Mn2+(aq) + 2e- → Mn(s)
-1.18
2H2O + 2e- → H2(g) + 2OH-(aq)
-0.83
Zn2+(aq) + 2e- →Zn(s)
-0.76
Cr3+(aq) + 3e- → Cr(s)
-0.74
Fe2+(aq) + 2e- → Fe(s)
-0.44
Cd2+(aq) + 2e- → Cd(s)
-0.40
PbSO4(s) + 2e- → Pb(s) + SO42-(aq)
-0.31
Co2+(aq) + 2e- → Co(s)
-0.28
Ni2+(aq) + 2e- → Ni(s)
-0.25
Sn2+(aq) + 2e- → Sn(s)
-0.14
Pb2+(aq) + 2e- →Pb(s)
-0.13
2H+(aq) + 2e- →H2(g)
0.00
Sn4+(aq) + 2e- →Sn2+(aq)
+0.13
Cu2+(aq) + e- →Cu+(aq)
+0.13
SO42-(aq) + 4H+(aq) + 2e- → SO2(g) + 2H2O
+0.20
AgCl(s) + e- →Ag(s) + Cl-(aq)
+0.22
Cu2+(aq) + 2e- →Cu(s)
+0.34
O2(g) + 2H2 + 4e- →4OH-(aq)
+0.40
I2(s) + 2e- →2I-(aq)
+0.53
MnO4-(aq) + 2H2O + 3e- →MnO2(s) + 4OH-(aq)
+0.59
O2(g) + 2H+(aq) + 2e- →H2O2(aq)
+0.68
Fe3+(aq) + e- →Fe2+(aq)
+0.77
Manual
42
US 244241
Rev.1 – Sept 08
Sparrow Consulting © September 08
Ag+(aq) + e- →Ag(s)
+0.80
Hg22+(aq) + 2e- →2Hg(l)
+0.85
2Hg2+(aq) + 2e- →Hg22+(aq)
+0.92
NO3-(aq) + 4H+(aq) + 3e- →NO(g) + 2H2O
+0.96
Br2(l) + 2e- →2Br-(aq)
+1.07
O2(g) + 4H+(aq) + 4e- →2H2O
+1.23
MnO2(s) + 4H+(aq) + 2e- →Mn2+(aq) + 2H2O
+1.23
Cr2O72-(aq) + 14H+(aq) + 6e- →2Cr3+(aq) + 7H2O
+1.33
Cl2(g) + 2e- →2Cl-(aq)
+1.36
Au3+(aq) + 3e- →Au(s)
+1.50
MnO4-(aq) + 8H+(aq) + 5e- →Mn2+(aq) + 4H2O
+1.51
Ce4+(aq) + e- →Ce3+(aq)
+1.61
PbO2(s) + 4H+(aq) + SO42-(aq) + 2e- →PbSO4(s) + 2H2O
+1.70
H2O2(aq) + 2H+(aq) + 2e- →2H2O
+1.77
Co3+(aq) + e- →Co2+(aq)
+1.82
O3(g) + 2H+(aq) + 2e- → O2(g) + H2O
+2.07
F2(g) + e- →F-(aq)
+2.87
Table 7: Reduction potentials at 25ºC
Whether the reaction will be oxidised or reduced is measured by the potentials listed in
Table 7. The standard reduction potentials make it possible for you to predict whether or
not the reaction will take place. The half reaction with a more positive potential always
undergoes reduction.
Thus, the other half reaction undergoes oxidation.
The more
negative the potential, the more difficult it is to bring about the half reaction.
One can determine if a redox reaction will take place under certain conditions by
calculating the voltage of the redox reaction. If it is positive, it implies that the reaction is
spontaneous. If the calculated voltage is negative it means that the reaction cannot occur
by itself.
Manual
43
US 244241
Rev.1 – Sept 08
Sparrow Consulting © September 08
Example
1
Is the following reaction spontaneous?
Hg + Mg2+ → Hg2+ + Mg
reduction: Mg2+ + 2e-→ Mg
E0 = -2.37 V
oxidation: Hg →Hg2+ + 2e-
E0 = 0.79 V
E ocell = E ocathode - E oanode
= -2.37 V- (+0.79)
= -3.16 V
2
Since the standard electrode potential is negative, this redox reaction is not
spontaneous. Thus energy will have to be applied for this reaction to take place.
In Table 7 the strength of reducing agents increases from the top to the bottom in the
table, while the strength of oxidising agents increases from the bottom to the top in the
table.
3.7
Experiment
The following experiment will be conducted in the chemistry laboratory so that you can
investigate a simple redox reaction.
Experiment
Observe a demonstration of and/ or conduct the following
experiment.
Purpose
To investigate a simple redox reaction
Apparatus and
materials
aqueous copper sulphate
zinc metal
2 test tubes
2 tweezers
25 ml graduated cylinder
small beaker
Manual
44
US 244241
Rev.1 – Sept 08
Sparrow Consulting © September 08
Safety hazards
and precautions
Procedure
Correct PPE, including eye protection should be worn at all
times throughout the experiment.
Obtain a small piece of zinc metal and record its appearance
specifically.
Carefully place the zinc into the test tube so that it rests at the
bottom.
Use the cylinder to measure 15 ml of CuSO4 solution and put it
into the small beaker.
Also record your observation of the colour of the CuSO4
solution.
Add 5 ml of the CuSO4 solution into the test tube and allow it to
rest. Record your observations during this period and again at
the end of the reaction.
Manual
45
US 244241
Rev.1 – Sept 08
Sparrow Consulting © September 08
UNIT 4: CHEMICAL REACTION RATES
4.1
Instructions
Ref. No
SO4 AC1-4
Resources
Learning materials
CCFO2-4, 6, 7
Learning Methodology
Read through Unit 4 of the learning materials.
You can either work individually or in pairs. Make
notes of things you do not understand and/ or
need more information on.
Workbook
Assessment
N/ a
N/ a
Act. 13
SO4 AC1-4
Classroom/ Laboratory
Attend a lecture on the:
CCFO1-7
Facilitator
principles of chemical reaction rates
Material and
equipment for
experiments:
factors affecting chemical reaction rates
principles of endothermic and exothermic
reactions
Computer and
projector with
animations of the
experiments and/ or
PowerPoint slides of
the experiments
industry applications demonstrating the role
of chemical reaction rates in industry.
During the lecture, you will observe and/ or
participate in experiments/ animations of the
experiments to help you understand the concept
of endothermic and exothermic reactions.
Ex. 7
Act. 14
Ass. 1
Ex. 8
Manual
46
US 244241
Rev.1 – Sept 08
Sparrow Consulting © September 08
Ref. No
Resources
SO4 AC4
On-site/ Classroom
CCFO1-7
Facilitator/ SME
Computer with internet
access
Learning Methodology
CCFO1-7
Learning materials and
workbook
PoE
Facilitator/ SME
Manual
Assessment
Ex. 9
Ass. 1
CCFOs
CCFOs
In pairs, do research on the application and effect
of chemical reaction rates in industry and collect
examples of the application of chemical reaction
rates in industry. Present your findings to the
class.
Act. 15
Multimedia for
presentation
SO4 AC1-4
Workbook
Revise the work that you have done up to this
point. Make sure that you have completed the
CCFO checklist and obtained the required
evidence for your PoE. If there is anything that
you do not understand, ask your facilitator.
47
Act. 16
US 244241
Rev.1 – Sept 08
Sparrow Consulting © September 08
4.1.1
Chemical reaction rates
A chemical reaction is a process in which chemical substances interact and conversion
takes place at a certain rate. The chemical substances in a reaction are referred to as
reactants. There are two types of chemical reactions namely endothermic and exothermic.
An endothermic reaction cannot occur spontaneously and thus require energy to proceed.
An exothermic reaction can occur spontaneously and releases energy in the form of heat,
light, or sound. The rate of chemical reactions is defined as the change in concentration of
a substance per time unit. The substance can be a reactant or the product. The reaction
rate can be best explained by the collisions theory, which states that a reaction can occur
when two atoms, ions or molecules collide with enough energy in a given time to form one
or more products. The more collisions in the system, the more combinations of molecules
will occur and the faster the reaction will happen. The reaction rate of this reaction is
higher.
Consider the following reaction:
A + B →C
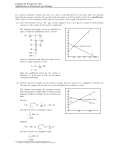
If one measures the concentration of A at a certain time interval, one typically sees a trend
as shown in the graph below.
Figure 7: Rate of reaction curve
This graph shows the concentration decreasing with time. Since this is not a straight line,
the slope (rate) of this graph changes with time, indicating that the reaction rate is not
constant. The rate is therefore related to the time the concentration measurements were
made.
Manual
48
US 244241
Rev.1 – Sept 08
Sparrow Consulting © September 08
4.1.2
Factors affecting the rate of reaction
There are several factors that can influence the chemical reaction rate. Generally, factors
that increase the number of collisions will increase the reaction rate, while those
decreasing the collisions will decrease the reaction rate. These factors are discussed
below:
4.1.2.1
Temperature
The increase in temperature will increase the kinetic energy in the reaction, which makes
the motion of particles more chaotic. Due to this, the number of collisions per unit time will
increase at higher temperatures.
4.1.2.2
Pressure
It is a known fact that atoms of a gas are very far apart. For two compounds to react there
must be contact between their molecules. By increasing the pressure, you force the
molecules to make contact with one another, which increase the frequency of collisions
between the particles. An increase in pressure also causes the temperature to rise. Thus
an increase in pressure of a gas results in an increase in chemical reaction rate.
4.1.2.3
Concentration
In a given volume, the number of reacting particles affects the reaction rate. An increase
in concentration is in essence an increase in reacting particles.
The higher the
concentration, the more collisions occur and therefore the faster the reaction rate will be.
4.1.2.4
Catalyst
A catalyst is a substance that speeds up the reaction rate without being consumed in the
reaction. Catalysts are widely used in the chemical industry to increase the reaction rate
for economical reasons. The presence of a catalyst decreases the energy required for the
reaction and therefore increasing the effective collisions.
4.1.2.5
Reaction surface area
If one of the reacting substances is a solid, its surface will affect the rate of chemical
reaction. A larger surface area will result in an increase of collisions between particles. It
is important to note that powders have a larger surface area than solids.
Manual
49
US 244241
Rev.1 – Sept 08
Sparrow Consulting © September 08
4.1.2.6
Reaction Energetics
For a chemical reaction to occur chemical bonds must be formed and others must be
broken. Energy is required to break these bonds. Some reactions require energy to
occur while others release energy in the process. Energy can be released in terms of
heat, light or sound.
Reactions that release energy are called exothermic reactions. The energy released by
these reactions is taken up in the environment. This may heat up the surroundings if
energy is released in form of heat. Exothermic reactions are mostly spontaneous and
explosive.
As a general rule, an increase in temperature results in an increase in
chemical reaction rate.
Once an exothermic reaction starts, the reaction rate quickly
increases due to the heat that is produced. Care must be taken when dealing with such
reactions.
The combustion of methane is an example of an exothermic reaction:
CH4 (g) + 2O2 (g) →CO2 (g) + 2H2O (g)
Generally exothermic reactions are written as:
Reactants →Products + energy
In an endothermic reaction, energy has to be supplied to the system continually in order
for the reaction to occur.
Photosynthesis is an example of an endothermic reaction.
Plants use energy from sunlight to convert water and carbon dioxide to produce oxygen
and glucose. This reaction is provided below:
Sunlight + 6CO2 (g) + H2O (l) = C6H12O6(aq) + 6O2(g)
In a general form, endothermic reactions are written as follows:
Reactants + energy →Products
4.1.2.7
Industrial applications of chemical reaction rates
The most important thing in industry is time, since time is money. The faster the reaction
rate of chemical reactions is, the more economic it is. It is important to know how long the
reaction will take to produce the desired products. This is why catalysts are used in the
chemical industry to increase reaction rates.
Reaction rates are studied for health and safety reasons. Fine powders have a large
surface area, which increases the reaction rate. This could cause an explosion if it is not
controlled well.
For example, methane gas and fine dust particles in mines are all
potentially dangerous.
Manual
Thus knowledge of the ignition temperature and explosion
50
US 244241
Rev.1 – Sept 08
Sparrow Consulting © September 08
threshold concentration of reactions helps us to design systems with minimal operational
risks.
Reaction rates are also used to design reactors.
4.2
Experiment
The following experiment will be conducted in the chemistry laboratory so that you can
investigate the factors that affect chemical reaction rates.
Experiment
Observe a demonstration of and/ or conduct the following
experiment.
Purpose
To investigate the factors that affect chemical reaction rates
Apparatus and
materials
8 x 0.1 g Magnesium strips
50 ml 1 M HCl solution
50 ml 2 M HCl solution
50 ml glass syringe
8 x test tube with rubber stoppers with holes at the top
elastic tube
stopwatch.
Correct PPE, including eye protection should be worn at all
Safety hazards
and precautions
times throughout the experiment.
In case of skin contact with HCl, flush the affected area with
lukewarm water for 15 minutes and seek medical attention.
Procedure
Construct the system as shown in the illustration.
Pour 10ml of the 1 M HCl solution into the test tube.
Add one 0.1 Mg strip to the edge of the test tube.
Plug the system and shake it to make the Mg strip contact the
HCl solution, start measuring the time with the stopwatch
immediately.
Repeat the experiment with the magnesium folded many time to
reduce the surface area.
Manual
51
US 244241
Rev.1 – Sept 08
Sparrow Consulting © September 08
Repeat the experiment with the Mg strip in the 2 M HCl solution.
Repeat the experiment with the Mg strip folded many times in
the 2M HCl solution.
Record the exact time each reaction took to complete.
Sketch (please
redraw)
Manual
52
US 244241
Rev.1 – Sept 08
Sparrow Consulting © September 08
UNIT 5: CHEMICAL EQUILIBRIUM
5.1
Instructions
Ref. No
SO5 AC1-5
Resources
Learning materials
CCFO2-4, 6, 7
Learning Methodology
Read through Unit 5 of the learning materials.
You can either work individually or in pairs. Make
notes of things you do not understand and/ or
need more information on.
Workbook
Assessment
N/ a
N/ a
Act. 17
SO5 AC1-4
Classroom/ Laboratory
Attend a lecture on:
CCFO1-7
Facilitator
The principles of phase equilibrium.
Material and
equipment for
experiments:
The principles of chemical equilibrium.
The factors affecting chemical equilibrium.
Equilibrium calculations involving
concentrations and the equilibrium constant.
Computer and
projector with
animations of the
experiments and/ or
PowerPoint slides of
the experiments
During the lecture, you will observe and/ or
participate in experiments/ animations of the
experiments to help you understand the concept
of chemical equilibrium.
Ex. 10
Act. 18
Ex. 11
Manual
53
US 244241
Rev.1 – Sep 08
Sparrow Consulting © September 08
Ref. No
Resources
SO5 AC5
On-site/ Classroom
CCFO1-7
Facilitator/ SME
Computer with internet
access
Learning Methodology
CCFO1-7
Learning materials and
workbook
PoE
Facilitator/ SME
Manual
Assessment
Ex. 12
Ass. 1
CCFOs
CCFOs
In pairs, do research on industry applications
demonstrating the role of chemical equilibrium
using practical industry examples.
Present your findings to the class.
Act. 19
Multimedia for
presentation
SO5 AC1-5
Workbook
Revise the work that you have done up to this
point. Make sure that you have completed the
CCFO checklist and obtained the required
evidence for your PoE. If there is anything that
you do not understand, ask your facilitator.
54
Act. 20
US 244241
Rev.1 – Sept 08
Sparrow Consulting © September 08
5.1.1
Equilibrium
Equilibrium is a fundamental concept in chemistry, so in this section we will explore the
application of equilibrium to chemical reactions.
In many cases, reactions do not reach completion, in other words there are still reactants
left at the end of the reaction. In some cases, the reactants are favoured over products
and vice versa. These are called reversible reactions. In such reactions, the conversion
of reactants to products and of products to reactants occurs at the same time. Reversible
reactions are indicated by double arrows.
As the reaction starts there are no products, therefore the reaction rate of the reverse
reaction is zero. The rate of the forward reaction decreases as the concentration of
reactants decrease, while the products build up. Since the concentration of the products
increases, the rate of the reverse reaction increases. Eventually the rate of reactants
converting to products will equal the rate of which products are converting to reactants.
This phenomenon is known as the chemical equilibrium. Chemical equilibrium is reached
when the rate of the forward reaction equals the reverse reaction.
At chemical
equilibrium, the concentration of both products and reactants stop changing with time but
are not equal.
For example, making of sulphur trioxide is a reversible reaction:
2SO2 (g) + O2 (g) ↔ SO3 (g)
Equilibrium implies that for every one molecule of oxygen, two molecules sulphur dioxide
that react two sulphur trioxide molecules will dissociate. Both the forward and reverse
reactions are illustrated in Figure 8. The graph shows that initially there was no SO3;
hence the reverse reaction could not proceed. As the concentration of SO 3 increases, the
reverse reaction rate increases while the forward rate decreases. This continues until
equilibrium is reached, when both reaction rates are equal.
Manual
55
US 244241
Rev.1 – Sept 08
Sparrow Consulting © September 08
Figure 8: Equilibrium of a trioxide producing reaction
5.1.2
Equilibrium Constant
Chemists use equilibrium constants instead of percentages to indicate the position of
equilibrium in a chemical reaction. Equilibrium constants relate to the concentration of
reactants and products at equilibrium at a given temperature.
Consider the hypothetical reaction, where A reacts with B to produce C and D at
equilibrium.
aA + bB ↔ cC + dD
The equilibrium constant: Keq = [C] c x [D] d / [A] a x [B] b
The important feature of the equilibrium expression is that:
K is dimensionless.
Concentrations of reactants always appear on the numerator.
Concentrations of products always appear on the denominator.
Concentrations are always raised to power of their stoichiometric coefficients.
Square brackets indicate the amount of a material in moles per litre.
It is important to note the following:
Equilibrium is temperature dependent. When the temperature changes, the constant
changes as well.
If the reagent is solid, its concentration is taken as 1 and does not appear on the
equilibrium constant expression.
Manual
56
US 244241
Rev.1 – Sept 08
Sparrow Consulting © September 08
For equilibrium reaction:
C(s) + CO2 (g) ↔ 2CO (g)
Keq = [CO]2 / [CO2]
Concentrations of solvents of dilute solutions are also taken as 1 and are omitted from the
Keq expression. For the reaction;
H2SO4 (aq) + H2O (l) ↔ HSO4-(aq) + H3O+ (aq)
Keq = [HSO4-] x [H3O+] / [H2SO4]
Example
1
What is the equilibrium constant expression for the following reaction?
4R + ½ S ↔ 3T + U
Keq = [R] 4 x [S] ½ / [T] 3x [U]
Example
1
A litre of a gas mixture at 450 oC at equilibrium contains 0.5 mol of hydrogen gas,
0.5 mol of iodine and 3.55 mol of hydrogen iodide. Determine the equilibrium
constant for the reaction t is the equilibrium constant expression for the following
reaction:
H2(g) + I2(g) ↔ 2HI(g)
Keq =
[HI]2 / [H2] x [I2]
=
(3.55 mol/l)2/ (0.5 mol/ l)x(0.5 mol/ l)
=
50.41
Example
1
One mol of hydrogen gas reacts with 1 mole iodine in a 1 litre flask at 450 oC. The
equilibrium, 1.8 mol of hydrogen iodide is present at equilibrium. Calculate the
Manual
57
US 244241
Rev.1 – Sept 08
Sparrow Consulting © September 08
equilibrium constant.
From the reaction:
H2(g) + I2(g) ↔ 2HI(g)
1.4 mol of HI implies that 0.7 mol of H2 and 0.7 mol of I2 were consumed in the
process. Thus at equilibrium:
mol I2 = mol H2 = 1 mol – 0.7mol
= 0.3 mol left at equilibrium
Therefore Keq
= [HI]2/ [H2] x [I2]
= (1.4)2/ (0.3)x(0.3)
= 21.78
Example
1
At a certain temperature, bromine chloride decomposes to form chlorine and
bromine and the equilibrium constant is 50. The equilibrium mixture contains 5mol
Br2 and 5mol Cl2. How many moles of bromine chloride are present at equilibrium
in a litre of a reactor?
For the reaction:
2BrCl (g) ↔ Br2(g) + Cl2(g)
Keq =
[Br2] x [Cl2] / [Br]2
Let y represent concentration of bromide chloride at equilibrium and solve for y:
50
= (5 mol/ l) x (5 mol/l)/ (y)2
y
= 0.707 mol/ l
Since the volume of the flask is 1 litre, moles of bromine chloride is 0.707mol.
Equilibrium constants give valuable information regarding the reactions. Amongst other
things, they give an indication of whether the reaction favours the reactants or products at
equilibrium. The equilibrium constant expression is always written as the ratio of products
to reactants. Therefore, when the value of Keq is greater than one it simply means that
there are more products than reactants at equilibrium. However, if Keq is less than one, it
implies that there are more reactants than products at equilibrium.
Therefore:
Manual
58
US 244241
Rev.1 – Sept 08
Sparrow Consulting © September 08
Keq
>1
Products are favoured at equilibrium.
Keq
<1
The expression gives a fraction, reactants are favoured at equilibrium.
5.1.3
Factors affecting chemical equilibrium
When a chemical system is in equilibrium, there is a delicate balance between reactants
and products. When stress is applied to such a system it can disturb the equilibrium. The
three most common ways in which equilibrium is disturbed are:
changes in temperature
changes in pressure
changes in concentration.
A change in any of the factors affecting the equilibrium conditions of a system will cause
the system to change in such a way that it cancels or reduces the effect of the change.
This is known as the Le Chatelier's Principle.
5.1.3.1
Effects of temperature changes
All chemical reactions are accompanied by an energy release or uptake. An increase in
temperature causes the equilibrium position to shift in to the half reaction that will absorb
heat. For example, an exothermic reaction producing SO3:
2SO2 (g) + O2 (g) ↔ SO3 (g) + heat
In this reaction, heat will be the by-product of the reaction. Suppose this reaction is at
equilibrium at temperature T1 and the temperature is increased to T2. According to Le
Chatelier, the net reaction will advance in the direction that will counteract this effect, thus
the equilibrium position will shift to the left and the reactants will be favoured.
For an endothermic reaction producing nitric oxide:
Heat + N2+ O2 ↔ 2NO
In this reaction, an increase in temperature will result in the equilibrium position shifting to
the right, producing more nitric oxide.
5.1.3.2
Effects of pressure changes
The effects of pressure are considered for gases only. For gases, an increase in pressure
will result in a decrease in volume, because volume and pressure are inversely
proportional.
Manual
59
US 244241
Rev.1 – Sept 08
Sparrow Consulting © September 08
A change in pressure only affects the equilibrium with an unequal number of moles of
products and reactants. The increasing pressure shifts the equilibrium position so that the
least number of moles form. For example, in the reaction:
N2 (g) + 3H2 (g) ↔ 2NH(g)
If pressure increases, the equilibrium will shift to the right, because there are 4 moles on
the left and only 2 on the right.
5.1.3.3
Effects of concentration changes
Changing the concentration of either the reactants or the products at equilibrium will result
in the reaction shifting to a new equilibrium position in order to counter the effect. Look at
the following example:
H2 + I2 ↔ 2HI
If you increase the concentration of hydrogen gas, the equilibrium will shift to the right. If
you remove some hydrogen gas, the equilibrium will shift to the left producing more
reactants.
Example
1
For the reaction: PCl5(g) + heat ↔ PCl3(g) + Cl2 (g)
How will the following affect the equilibrium?
Addition of PCl5
Increase in pressure
Removal of heat
Removal of Cl2
Addition of PCl5 will cause the equilibrium position to shift to the right, giving more
products.
An increase in pressure will shift the equilibrium position to the side with fewer
molecules. Thus it will shift to the left, favouring the reactants.
Removing heat will result in the equilibrium position shifting to the left, decreasing
the products at equilibrium.
Removal of Cl2 causes a shift to the right to produce more Cl2.
Manual
60
US 244241
Rev.1 – Sept 08
Sparrow Consulting © September 08
5.1.3.4
Industry applications of chemical equilibrium
To maximise profits in the process industry, the amount of products manufactured need to
be as high as possible and the ‘leftover’ reactants as low as possible. Le Chatelier’s
principle and the other principles of reaction kinetics can be used to design reactions that
are economical and produce the highest amounts of product and the lowest amount of byproducts.
5.2
Experiment
The following experiment will be conducted in the chemistry laboratory so that you can
investigate shifting of equilibrium using Le Chatelier’s principle.
Experiment
Observe a demonstration of and/ or conduct the following
experiment.
Purpose
To investigate shifting of equilibrium using Le Chatelier’s principle.
Apparatus and
materials
test tubes
nitrogen dioxide is prepared by your facilitator in a fume hood
and placed in a test tube with a stopper on top
Safety hazards
and precautions
ice and water bath
hot water bath.
The first part of the experiment should be conducted in a fume
cupboard.
Correct PPE, including eye protection should be worn at all
times throughout the experiment.
Procedure
Record your observation of the colour of the gas in the test
tube.
Place the test tube filled with nitrogen dioxide in the ice and
water bath for five to six minutes.
Record your observations after this time.
Place the test tube in hot water bath for the same amount of
time and record your observations.
Manual
61
US 244241
Rev.1 – Sept 08
Sparrow Consulting © September 08
ANNEXURE 1: RESOURCES
Primary Resources
http://www.wpbschoolhouse.btinternet.co.uk/ page07/ equilibria3.htm
www.sasked.gov.sk.ca/ docs/ chemistry/ unit8chem30.html
ANNEXURE 2: ICONS
Checklist
Demonstration
Example
Group work/ discussion
Important Note/ Something to think about
Lecture
Multimedia
Experiment
Practical exercise/ assessment
Safety & PPE
Manual
62
US 244241
Rev.1 – Sept 08
Sparrow Consulting © September 08
Self-study
Site visit
Written exercise
This manual was developed by
Sparrow Research and Industrial Consultants CC
e-mail: [email protected]
Tel: 012 – 460 9755
ANNEXURE 3: US 244075
SOUTH AFRICAN QUALIFICATIONS AUTHORITY
REGISTERED UNIT STANDARD:
Apply knowledge of chemical reactions in a processing environment.
SAQA US ID
UNIT STANDARD TITLE
244241
Apply knowledge of chemical reactions in a processing
environment.
SGB NAME
REGISTERING PROVIDER
Chemical Industries SGB
FIELD
SUBFIELD
Field 06 - Manufacturing, Engineering and
Technology
Engineering and Related Design
ABET BAND
UNIT STANDARD
TYPE
NQF LEVEL
CREDITS
Undefined
Regular
3
6
REGISTRATION
STATUS
REGISTRATION
START DATE
REGISTRATION
END DATE
SAQA DECISION
NUMBER
Registered
2007-06-27
2010-06-27
SAQA 0371/07
LAST DATE FOR ENROLMENT
Manual
LAST DATE FOR ACHIEVEMENT
63
US 244241
Rev.1 – Sept 08
Sparrow Consulting © September 08
2011-06-27
2014-06-27
This unit standard does not replace any other unit standard and is not replaced by any
other unit standard.
PURPOSE OF THE UNIT STANDARD
Learners who demonstrate competence as described in the outcomes of this unit standard
will be able to understand elementary chemistry and its application in the process
industry. The qualifying learner is able to:
Perform elementary chemical calculations.
Demonstrate an understanding of acids and bases.
Demonstrate an understanding of oxidation-reduction (redox) reactions and their
industrial applications.
Demonstrate understanding of chemical reaction rates.
Demonstrate understanding of chemical equilibrium.
LEARNING ASSUMED TO BE IN PLACE AND RECOGNITION OF PRIOR LEARNING
National Certificate Chemical Operations NQF Level 2 or equivalent, including
communication and mathematical literacy, NQF Level 2.
UNIT STANDARD RANGE
The typical context of this unit standard includes any processing environment, for example
chemical, minerals or beverage processing.
Range statements, which are applicable to the unit standard titles, specific outcomes and
assessment criteria are found beneath the applicable assessment criteria.
Specific Outcomes and Assessment Criteria:
SPECIFIC OUTCOME 1
Perform elementary chemical calculations.
ASSESSMENT CRITERIA
ASSESSMENT CRITERION 1
The concepts of atomic number, mass number, atomic mass, mole and Avogadro
constant are defined and explained in terms of scientific principles.
ASSESSMENT CRITERION 2
The relative atomic masses for a range of elements are calculated with information
obtained from the periodic table of elements.
ASSESSMENT CRITERION 3
The relative formula masses for a range compounds are calculated with information
obtained from the periodic table of elements.
ASSESSMENT CRITERION 4
The term "isotope" is defined and explained in terms of an atomic model.
ASSESSMENT CRITERION 5
Manual
64
US 244241
Rev.1 – Sept 08
Sparrow Consulting © September 08
The masses for a mole of different substances are calculated by using accepted scientific
principles.
ASSESSMENT CRITERION 6
The volumes for a mole of different gases are calculated by using accepted scientific
principles.
ASSESSMENT CRITERION 7
The mass and percentages of substances are calculated using chemical formulas and
balanced chemical equations.
SPECIFIC OUTCOME 2
Demonstrate an understanding of acids and bases.
ASSESSMENT CRITERIA
ASSESSMENT CRITERION 1
The properties of acids and bases are defined and explained using chemical terminology.
ASSESSMENT CRITERION 2
Strong and weak acids are defined and explained in terms of the acid and base
equilibrium constants.
ASSESSMENT CRITERION 3
The ionisation of water and the concept of pH are explained in terms of the ionisation
constant for water.
ASSESSMENT CRITERION 4
A list of acids is made in order of descending strength by using the ionisation constant for
water for each acid.
ASSESSMENT CRITERION 5
A range of acid reactions are balanced and explained by using accepted chemical
techniques and notation.
ASSESSMENT CRITERION RANGE
Included are reactions with metals, metal oxides, metal hydroxides, carbonate and alkalis.
ASSESSMENT CRITERION 6
Standardisation calculations are performed using basic scientific principles.
SPECIFIC OUTCOME 3
Demonstrate an understanding of oxidation-reduction (redox) reactions and their industrial
applications.
ASSESSMENT CRITERIA
ASSESSMENT CRITERION 1
The principles of reduction and oxidation reactions are explained in terms of electron
transfer principles.
ASSESSMENT CRITERION 2
The principles of standard electrode potentials are explained with the assistance of a table
with standard electrode potentials.
ASSESSMENT CRITERION 3
Manual
65
US 244241
Rev.1 – Sept 08
Sparrow Consulting © September 08
A range of redox reactions are written down and balanced using scientific principles and
notation.
ASSESSMENT CRITERION 4
Redox reactions are balanced by using the oxidation states of the elements.
ASSESSMENT CRITERION 5
A prediction can be made whether redox reactions will take place based on information
obtained from a redox table.
SPECIFIC OUTCOME 4
Demonstrate understanding of chemical reaction rates.
ASSESSMENT CRITERIA
ASSESSMENT CRITERION 1
The principles of chemical reaction rates are described in terms of scientific principles and
terminology.
ASSESSMENT CRITERION 2
The factors affecting chemical reaction rates are explained with the assistance of practical
examples.
ASSESSMENT CRITERION RANGE
Factors include but are not limited to the nature of substances involved in the reaction,
reaction surface area, concentrations, temperature and presence of a catalyst.
ASSESSMENT CRITERION 3
The principles of endothermic and exothermic reactions are described in terms of
scientific principles and terminology.
ASSESSMENT CRITERION 4
Industry applications demonstrating the role of chemical reaction rates are described
using practical industry examples.
SPECIFIC OUTCOME 5
Demonstrate understanding of chemical equilibrium.
ASSESSMENT CRITERIA
ASSESSMENT CRITERION 1
The principles of phase equilibrium are described in terms of scientific principles and
terminology.
ASSESSMENT CRITERION 2
The principles of chemical equilibrium are described in terms of the equilibrium constant.
ASSESSMENT CRITERION 3
The factors affecting chemical equilibrium are explained with the assistance of Le
Chatelier`s Principle.
ASSESSMENT CRITERION RANGE
Factors include but are not limited to concentration, pressure and temperature.
ASSESSMENT CRITERION 4
Manual
66
US 244241
Rev.1 – Sept 08
Sparrow Consulting © September 08
Equilibrium calculations are performed involving concentrations and the equilibrium
constant.
ASSESSMENT CRITERION 5
Industry applications demonstrating the role of chemical equilibrium are described using
practical industry examples.
UNIT STANDARD ACCREDITATION AND MODERATION OPTIONS
An assessor, accredited with a relevant NQF Level 3 or higher qualification, will assess
the learner’s competency.
Only an assessor with considerable first hand experience in process operations will
assess the learner’s competency.
Anyone assessing a learner or moderating the assessment of a learner against this
qualification must be registered as an assessor with the relevant ETQA.
Direct observation in simulated or actual work conditions is required.
UNIT STANDARD ESSENTIAL EMBEDDED KNOWLEDGE
The qualifying learner understands and is able to:
Perform elementary chemical calculations.
Demonstrate an understanding of acids and bases.
Demonstrate an understanding of oxidation-reduction (redox) reactions and their
industrial applications.
Demonstrate understanding of chemical reaction rates.
Demonstrate understanding of chemical equilibrium.
UNIT STANDARD DEVELOPMENTAL OUTCOME
N/ A
UNIT STANDARD LINKAGES
N/ A
Critical Cross-field Outcomes (CCFO):
1. UNIT STANDARD CCFO IDENTIFYING
Identify and solve problems in the following areas, which response displays that
responsible decisions, using critical and creative thinking, have been made:
Refer to all Specific Outcomes.
2. UNIT STANDARD CCFO ORGANISING
The learner is able to organise and manage himself and his activities responsibly and
effectively.
Refer to all Specific Outcomes.
3. UNIT STANDARD CCFO COLLECTING
Collect, analyse, organise and critically evaluate information by:
Refer to all Specific Outcomes.
Manual
67
US 244241
Rev.1 – Sept 08
Sparrow Consulting © September 08
4. UNIT STANDARD CCFO COMMUNICATING
Communicate effectively by using mathematical and/or language skills in the modes of
oral and/ or written presentations during:
Refer to all Specific Outcomes.
5. UNIT STANDARD CCFO SCIENCE
Use science and technology effectively and critically, showing responsibility towards the
environment and health of others when:
Refer to all Specific Outcomes.
6. UNIT STANDARD CCFO DEMONSTRATING
Demonstrate an understanding of the world as a set of related systems. This is evident in:
Refer to the following Specific Outcome/ s:
Demonstrate understanding of chemical reaction rates.
Demonstrate understanding of chemical equilibrium.
7. UNIT STANDARD CCFO CONTRIBUTING
Contribute to the full personal development of each learner and the social and economic
development of the society at large by:
Refer to all Specific Outcomes.
ANNEXURE 4: GLOSSARY
You will often encounter words, acronyms or abbreviations that you do not understand. It
is important that you familiarise yourself with industry-specific terms as soon as possible.
Abbreviations, acronyms and terminology:
Term
Description
A
Atomic number
The number of protons in the nucleus of an atom.
C
Corrosion
To destroy a metal or an alloy gradually by a process called
oxidation or by a chemical reaction
Electrochemical cell
A cell where the reaction occurs spontaneously and an
electrolytic cell where a non-spontaneous reaction occurs.
Endothermic
reaction
A reaction where energy has to be supplied to the system
continually in order for the reaction to occur.
Equilibrium
constant
Chemists use equilibrium constants instead of percentages to
indicate the position of equilibrium in a chemical reaction.
Equilibrium constants relate the concentration of reactants
and products at equilibrium at a given temperature.
E
Manual
68
US 244241
Rev.1 – Sept 08
Sparrow Consulting © September 08
Isotopes
Atoms that have the same atomic number but a different
atomic mass. They have the same number of protons and
electrons but different number of neutrons.
Mass number
The sum of the protons and neutrons in the nucleus.
Oxidise
A chemical reaction where oxygen is added or combined to a
substance and an oxide is formed
Oxidation number
The charge an atom would have if bonding electrons were
transferred completely to the atom with greater affinity for
them in a particular situation.
Rate of chemical
reaction
The change in concentration of a substance per time unit.
Relative atomic
mass
The ratio of the absolute mass of an atom of that element to
that of the atomic mass unit.
Reduction-oxidation
(redox)
Reactions are a group of reactions that are concerned with
transfer of electrons between compounds.
I
M
O
R
Manual
69
US 244241